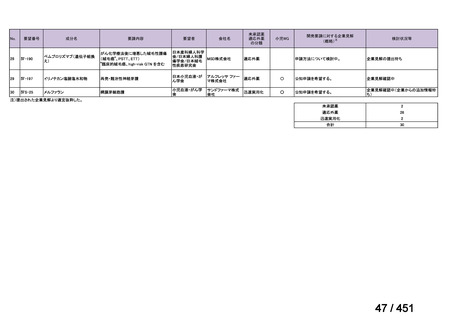
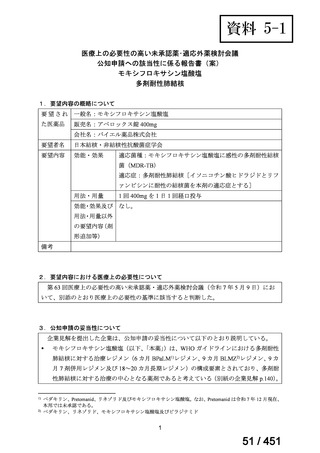
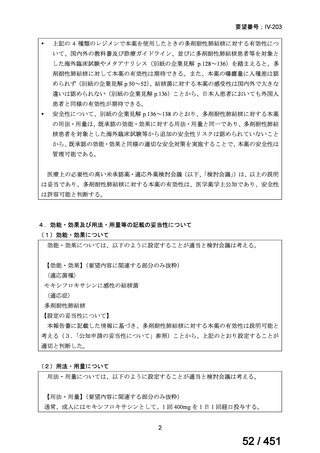
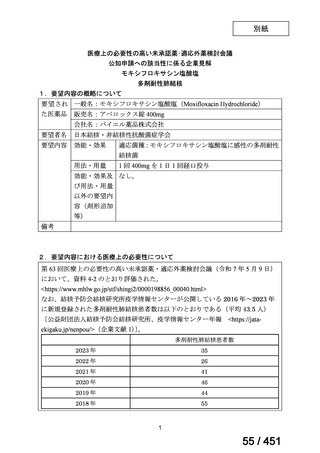
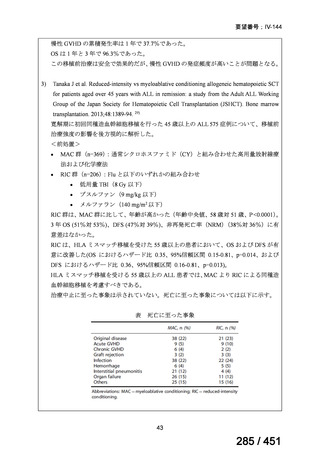
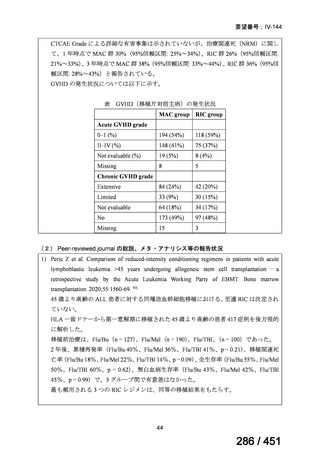
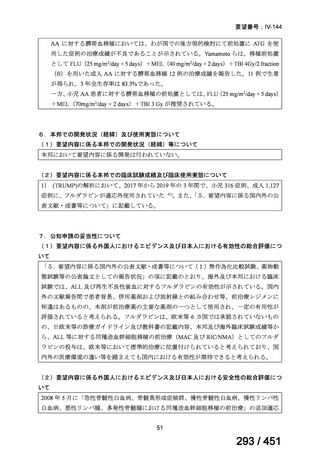
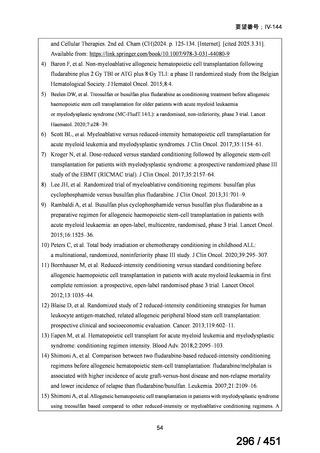
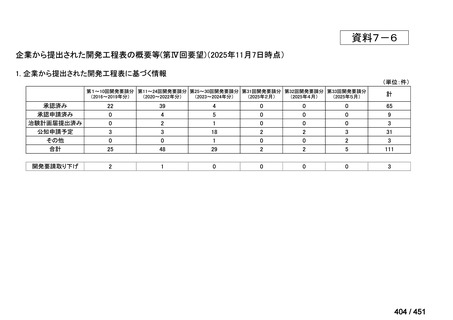
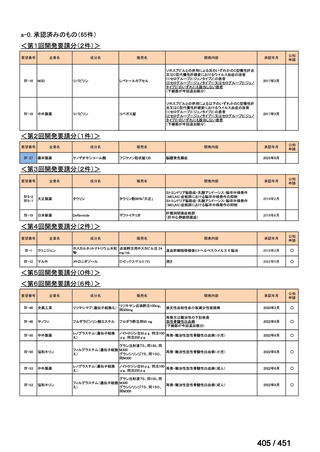
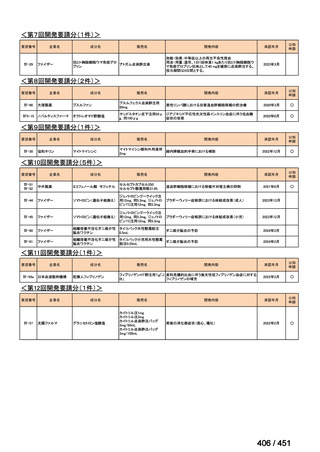
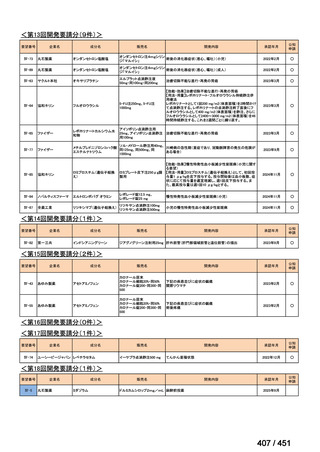
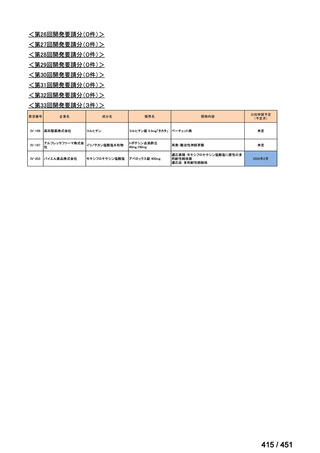
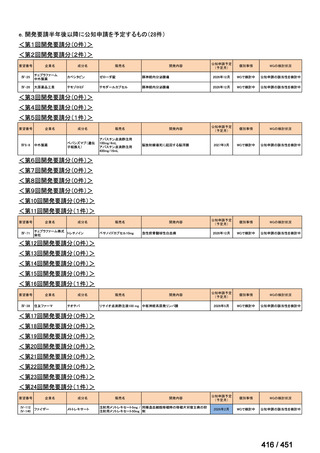
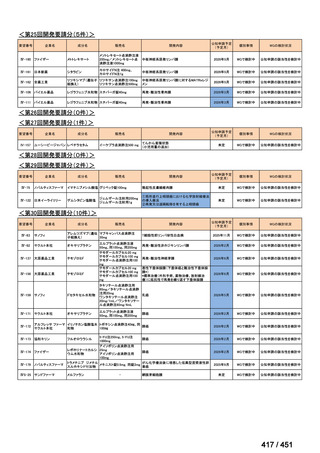
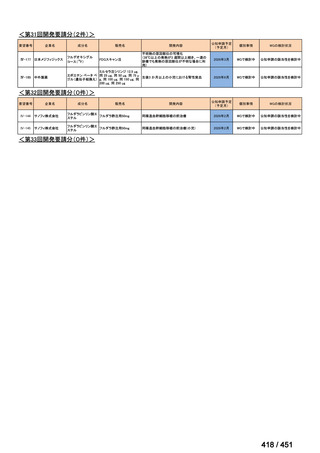
会議資料 (171 ページ)
出典
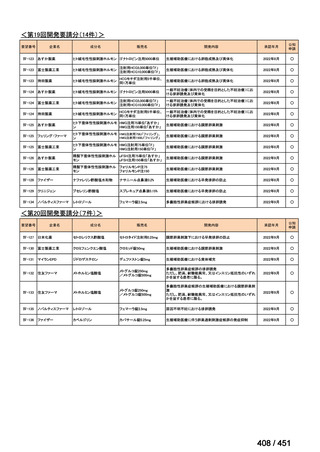
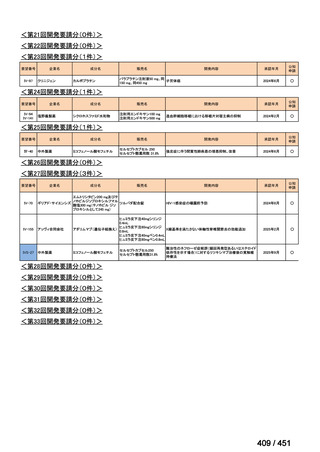
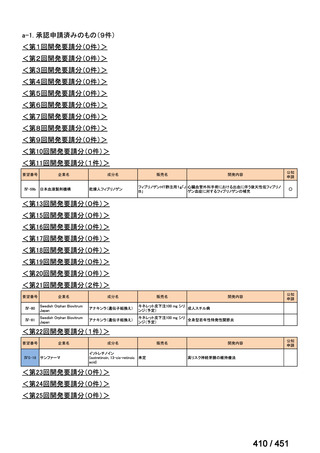
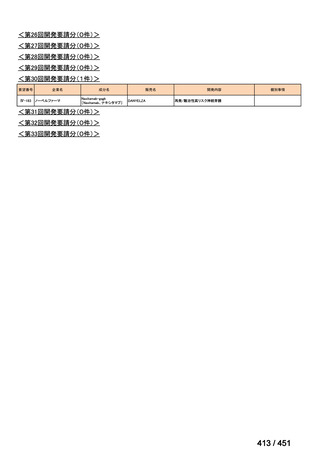
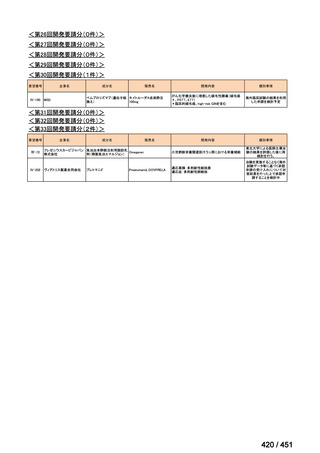
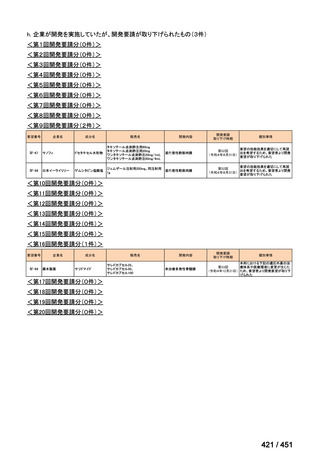
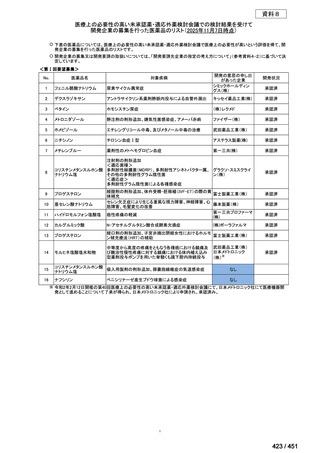
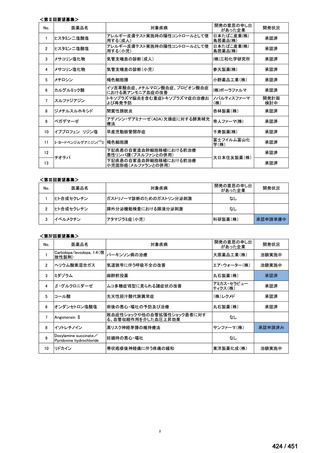
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00044.html |
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第66回 12/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
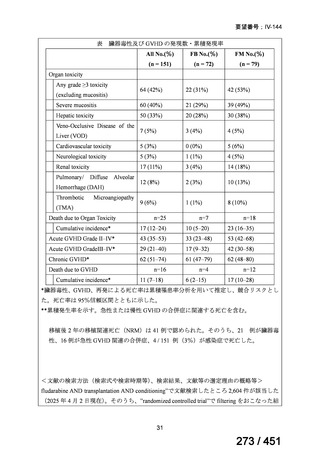
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
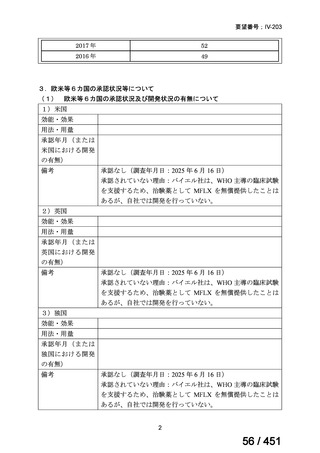
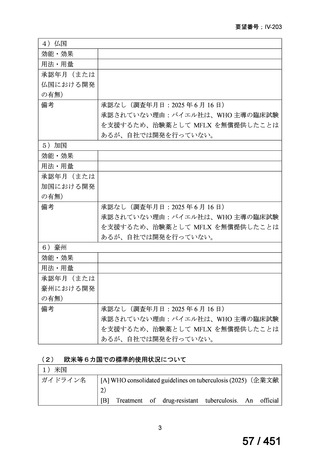
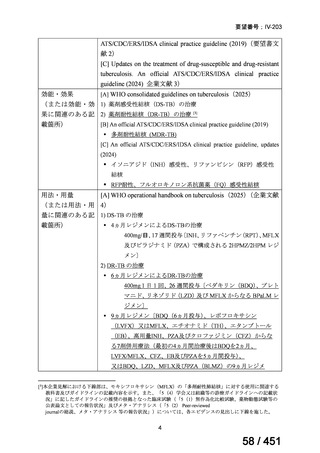
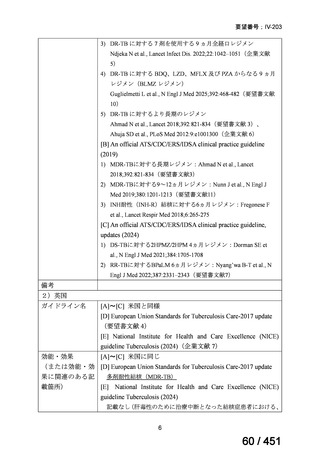
On current evidence, use of the BPaLM and BPaL regimens is limited to patients who:
are 15 years and older
do not have severe extra-pulmonary disease (miliary TB, TB meningitis,
osteoarticular TB or pericardial TB)
are not pregnant or breastfeeding
have not had previous exposure to bedaquiline, pretomanid, or linezolid
for greater than one month.
If the above regimens cannot be implemented due to not meeting the above criteria or
in full due to adverse effects or drug interactions, a longer all-oral regimen is indicated.
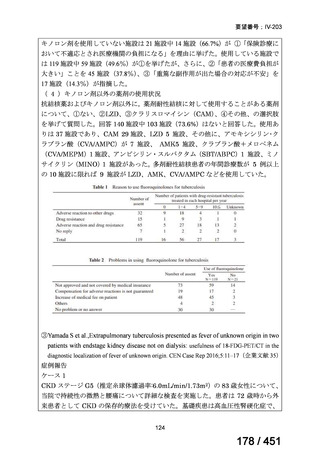
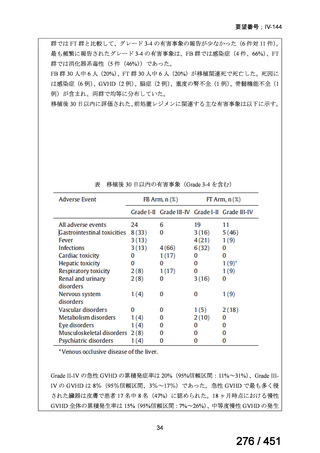
3. 9–11 month all-oral regimen:
Although this standardised shorter course regimen is still a WHO option that can be
considered for use, the inclusion of drugs with proven or possible resistance such as
isoniazid, ethionamide and pyrazinamide, has raised concerns. The
ATS/CDC/ERS/IDSA guideline (2019) did not make a recommendation for or against
the use of this regimen.
The regimen comprises
an initial phase: 4–6 months bedaquiline (6 months), moxifloxacin or
levofloxacin, clofazimine, ethionamide (or linezolid 2 months), isoniazid
(high dose), ethambutol, pyrazinamide then
a continuation phase: 5 months moxifloxacin or levofloxacin,
clofazimine, ethambutol, pyrazinamide;
linezolid (600 mg daily) for an initial 2 months can be considered as an
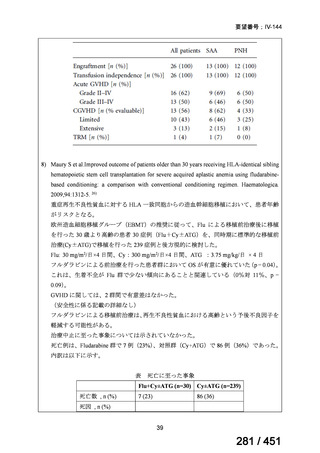
alternative to ethionamide for 4 months;
extension of the initial phase of treatment to 6 months will depend on
clinical and bacteriological assessment.
This shorter course all-oral regimen should only be considered in those with:
confirmed fluoroquinolone susceptibility;
non-extensive pulmonary disease (no bilateral cavitary or extensive
parenchymal disease on chest radiology) or non-severe extra-pulmonary
disease (no miliary TB, TB meningitis, osteoarticular TB or pericardial
TB);
for children less than 15 years of age, other extra-pulmonary sites are also
excluded (except lymph peripheral nodes or isolated mediastinal mass
without compression);
no additional resistance to other first or second line drugs (other than
isoniazid; if a katG mutation is present, high dose isoniazid is unlikely to
be of benefit) or previous use of any drugs contained in the regimen for
greater than one month.
Note: Ethionamide (or prothionamide) is contra-indicated in pregnancy. This 9–
11 month oral regimen should only be considered in pregnancy if ethionamide is
replaced with linezolid
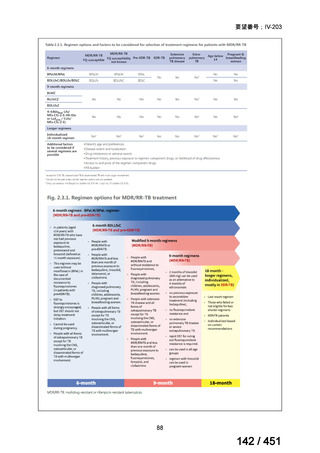
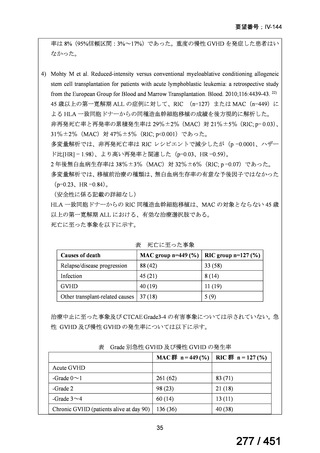
4. All-oral longer course regimen
The use of a longer course individualised regimen should be considered in those
with more extensive forms of disease, or if a shorter course regimen cannot be used
because eligibility criteria are not met or treatment is failing or drug intolerance issues
arise.
The design of the regimen is based on a priority selection of drugs from the new WHO
drug groupings (see table 1 below) which should be supported by drug susceptibility
testing (DST) and careful pre-treatment evaluation of the patient. Minor differences
between the WHO (2019) and ATS/CDC/ERS/IDSA (2019) guidelines include:
Initial drug selection in fluoroquinolone susceptible cases should include
at least 4 drugs from WHO groups A and B, consider 5 (WHO);
ATS/CDC/ERS/IDSA advise at least 5 drugs.
117
171 / 451