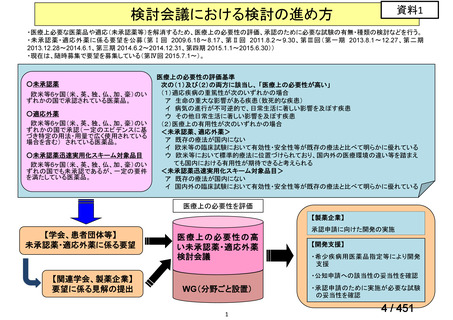
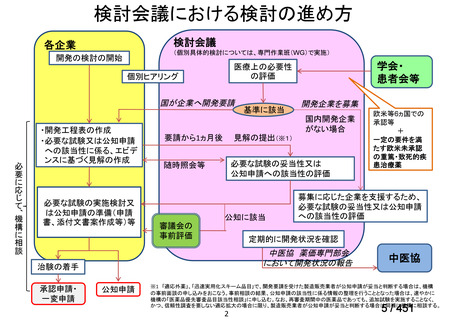
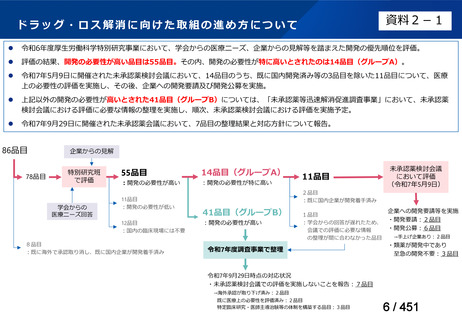
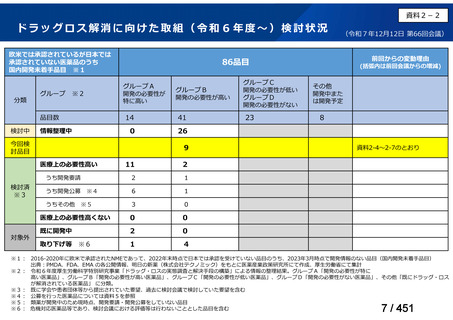
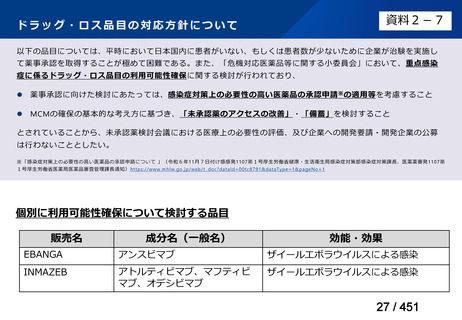
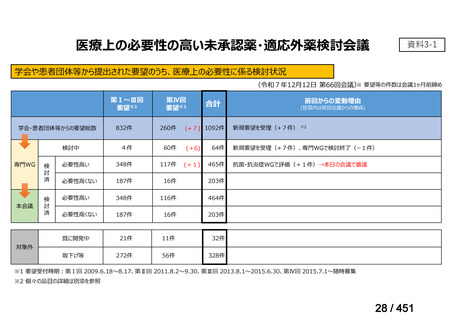
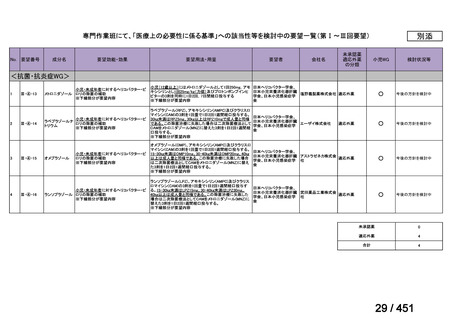
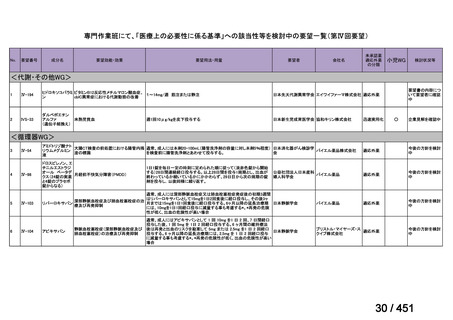
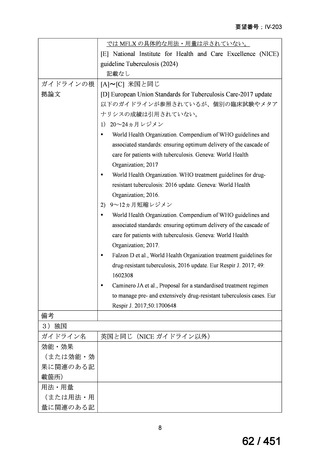
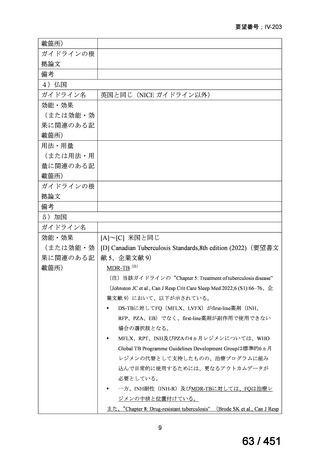
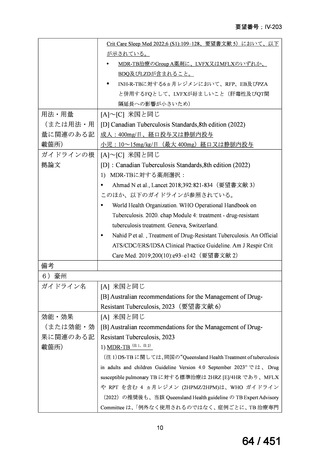
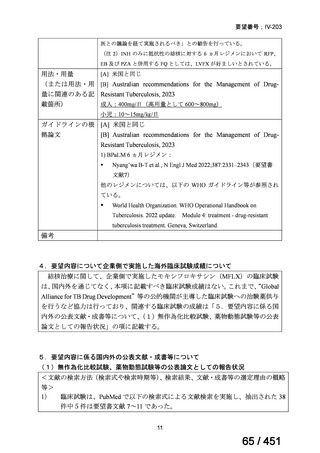
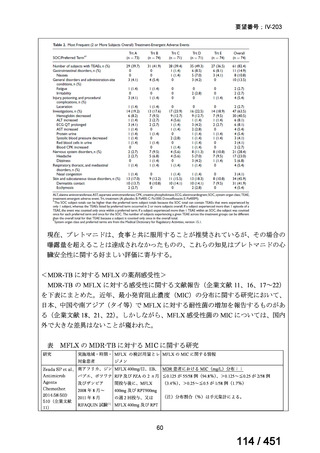
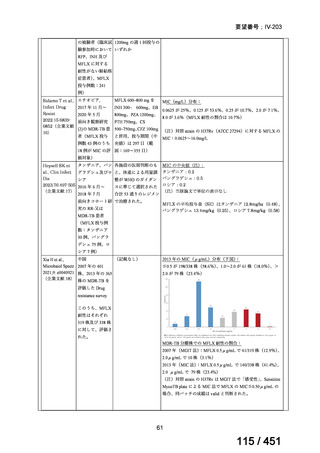
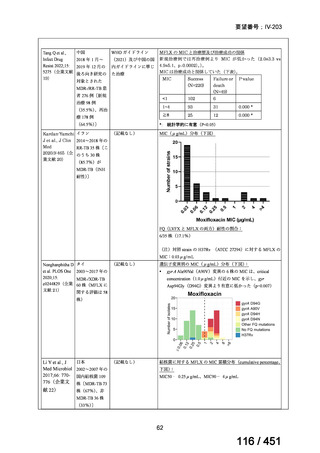
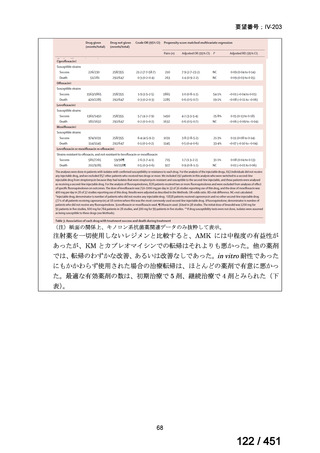
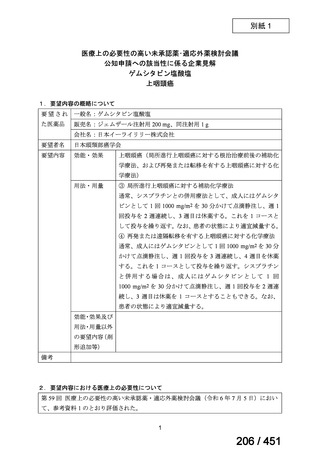
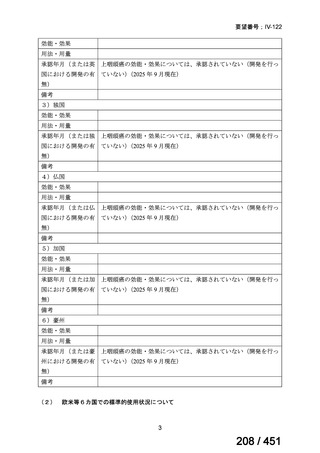
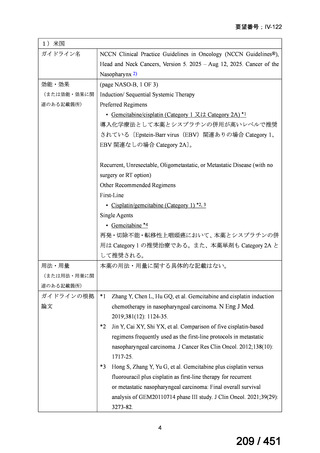
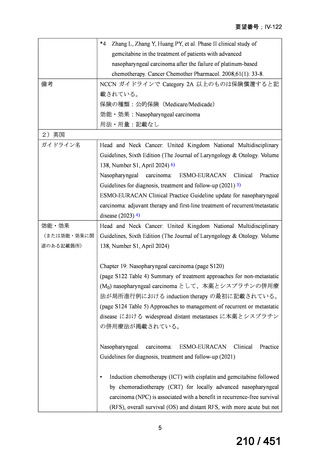
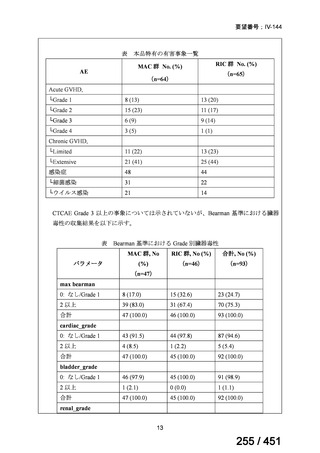
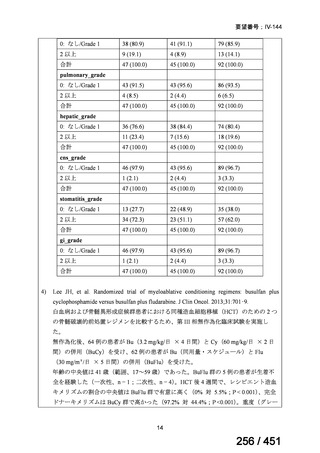
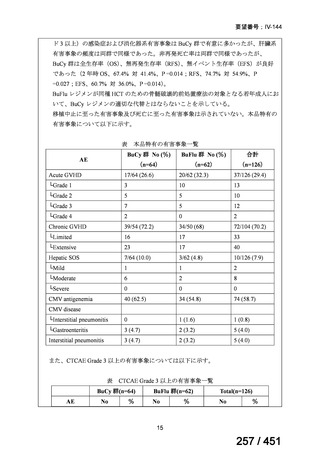
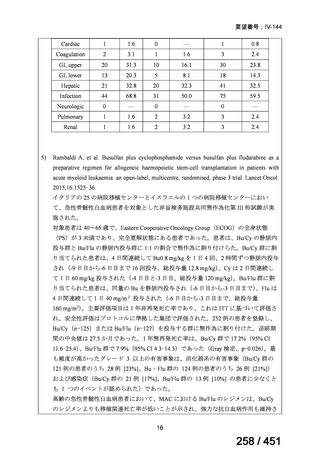
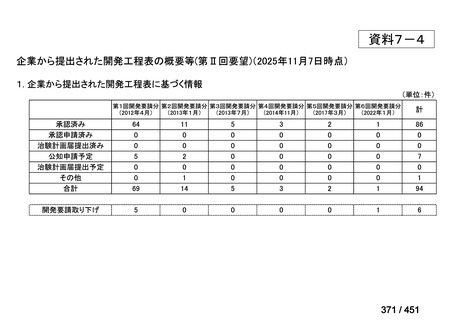
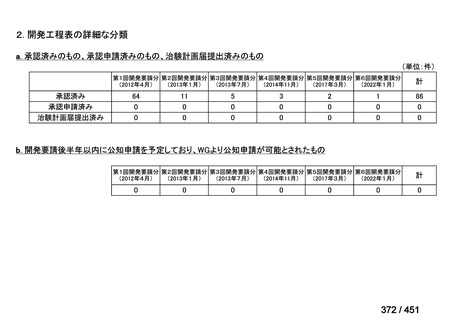
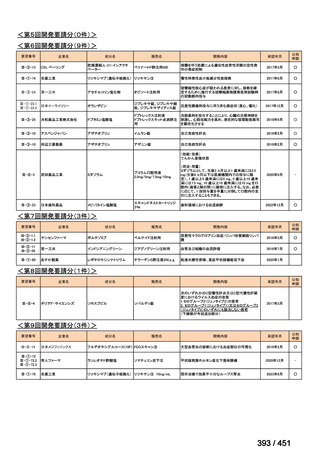
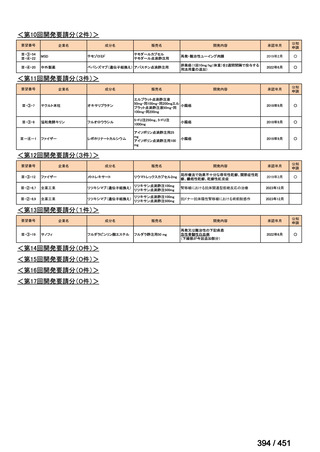
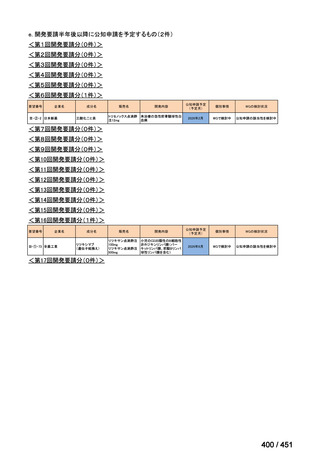
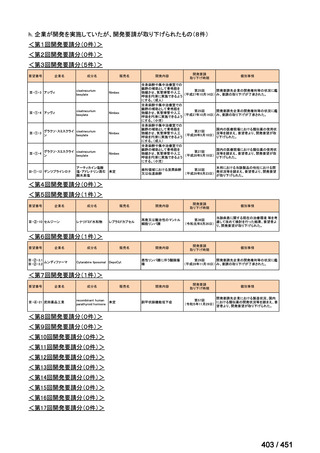
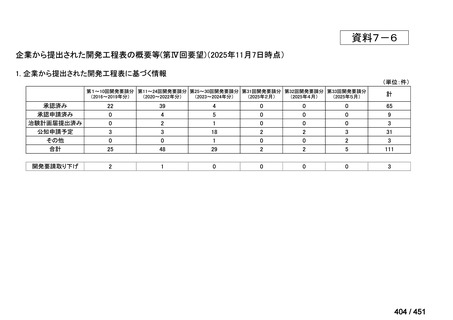
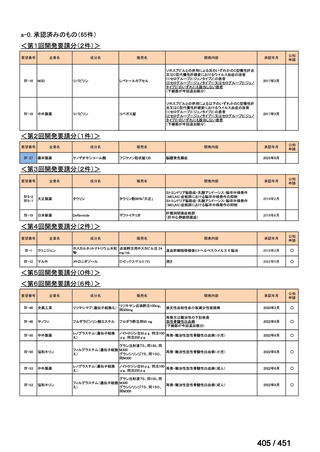
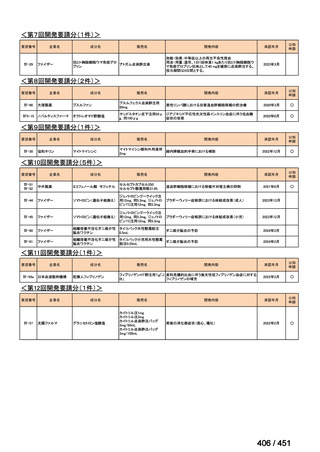
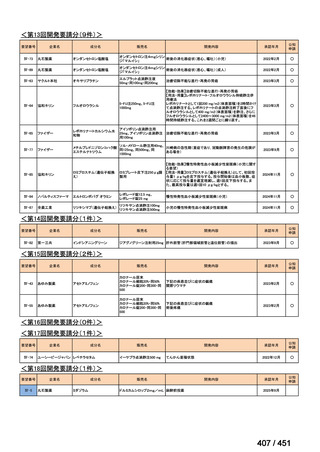
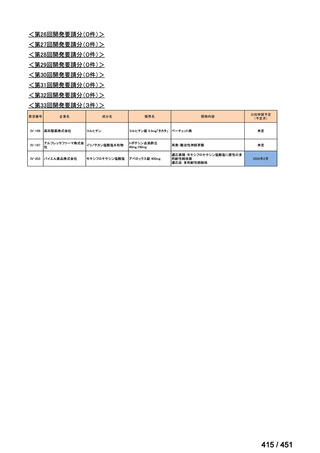
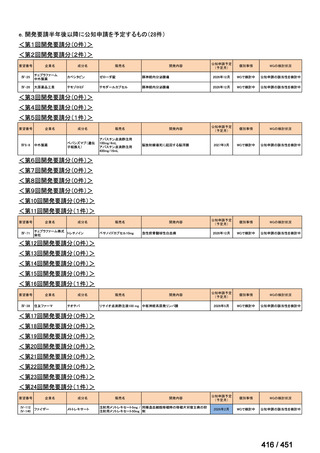
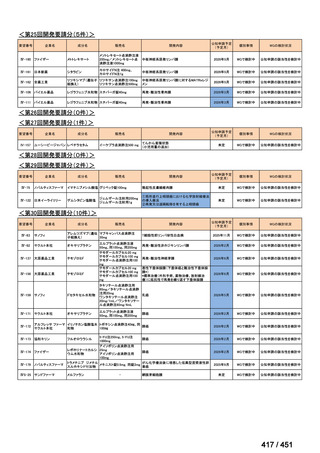
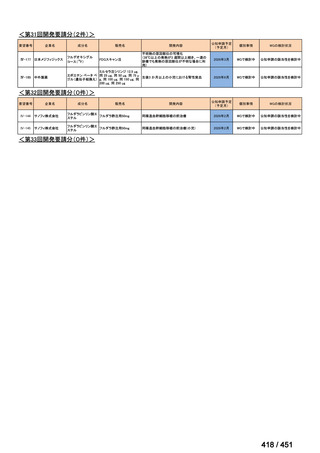
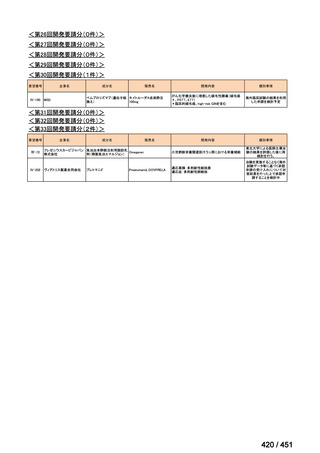
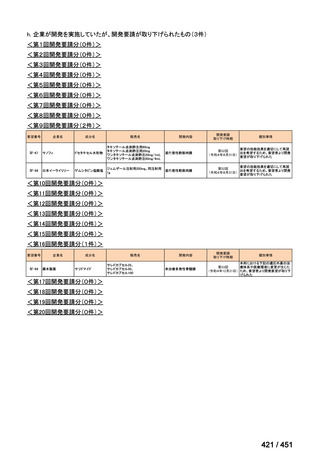
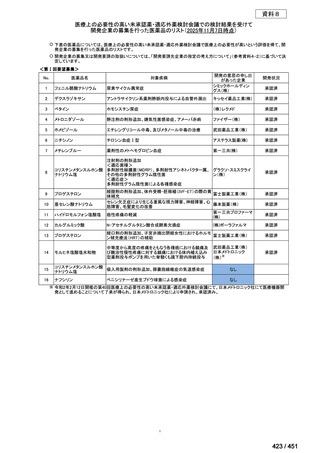
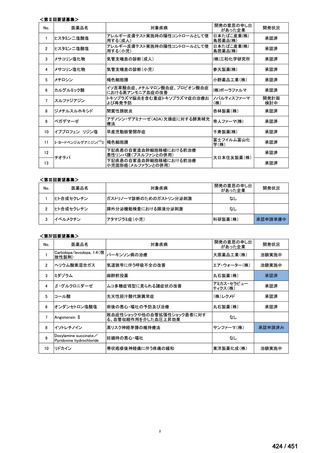
会議資料 (155 ページ)
出典
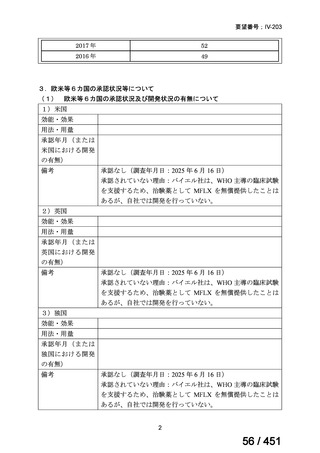
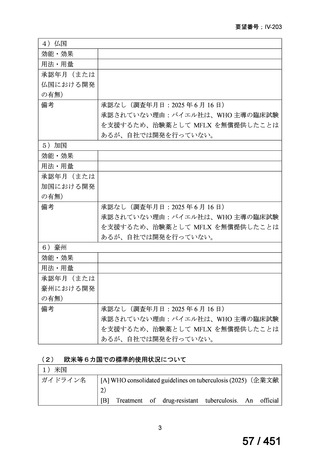
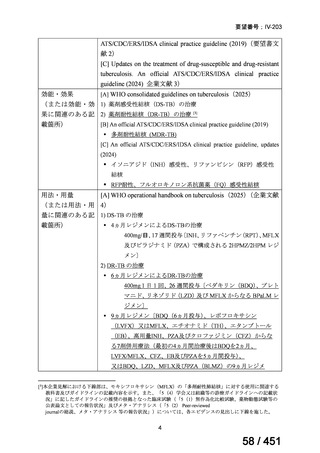
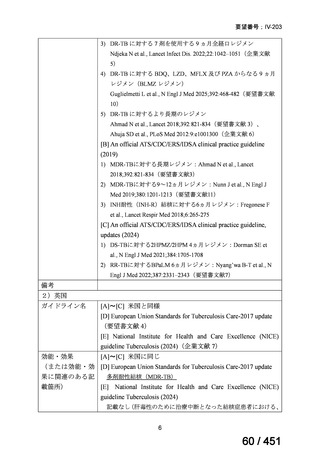
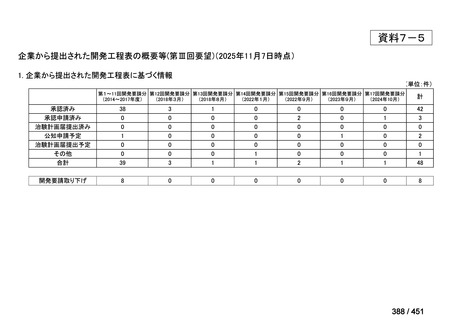
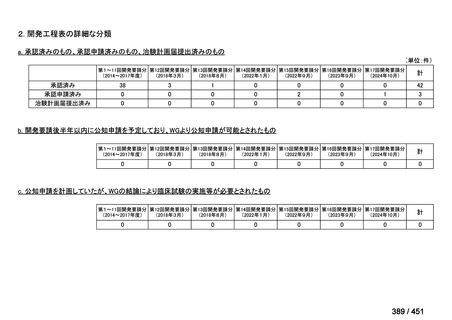
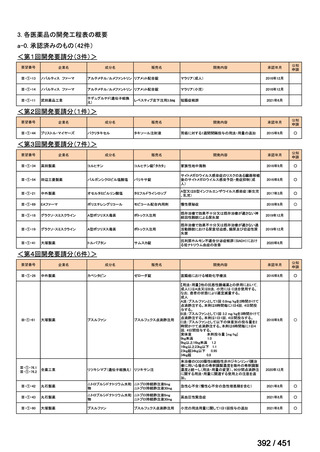
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00044.html |
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第66回 12/12)《厚生労働省》 |
ページ画像
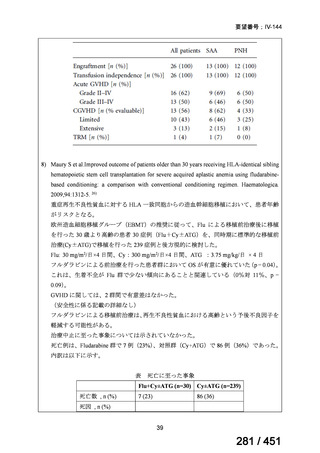
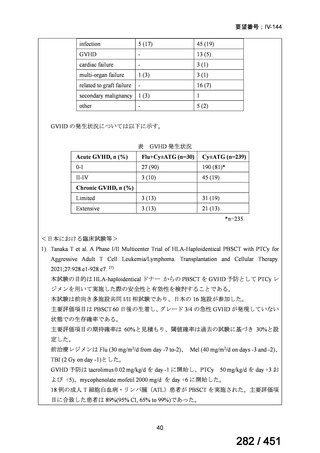
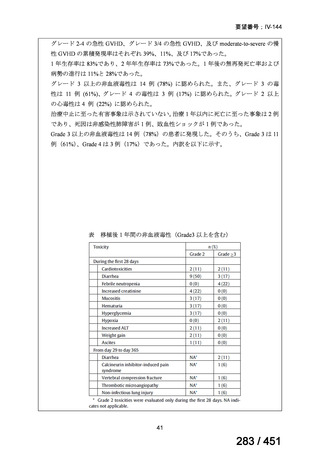
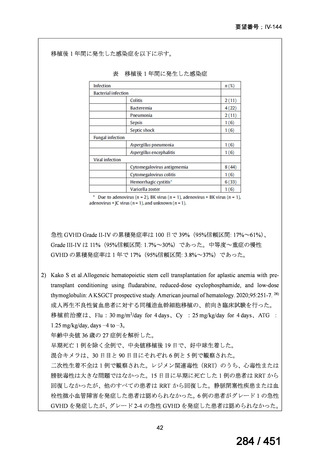
プレーンテキスト
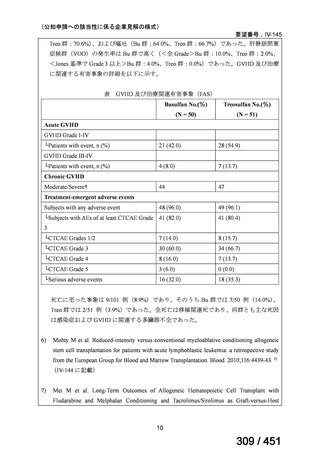
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
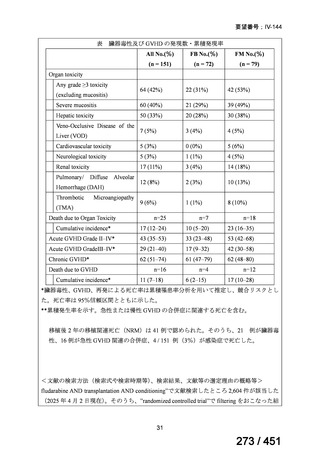
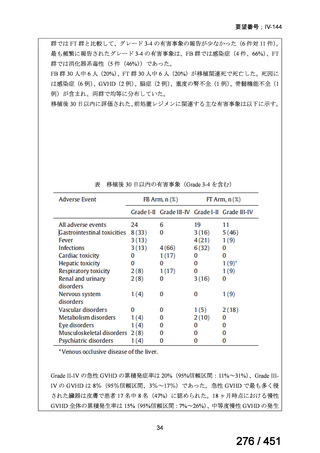
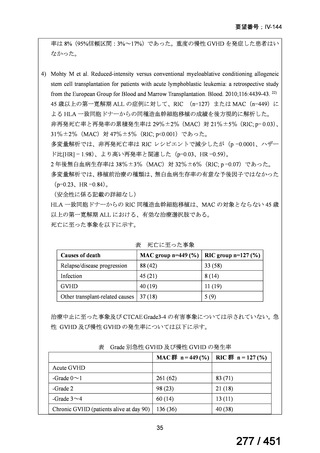
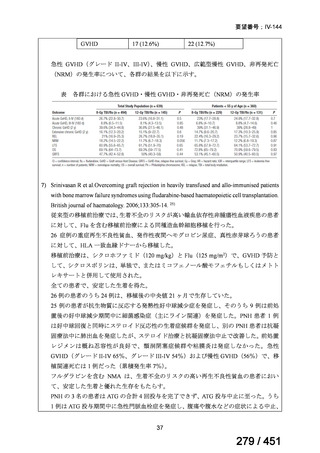
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
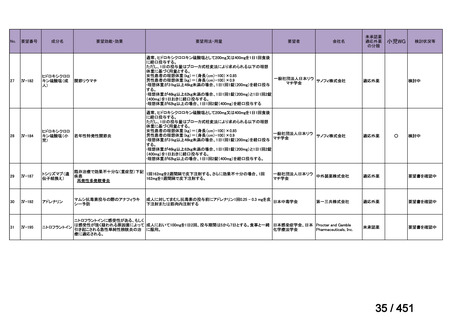
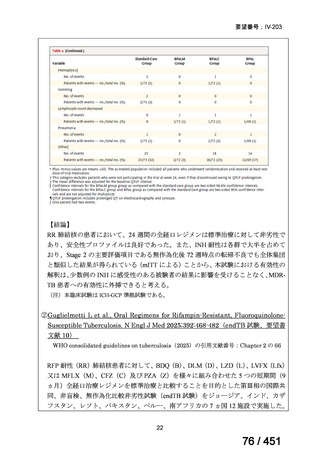
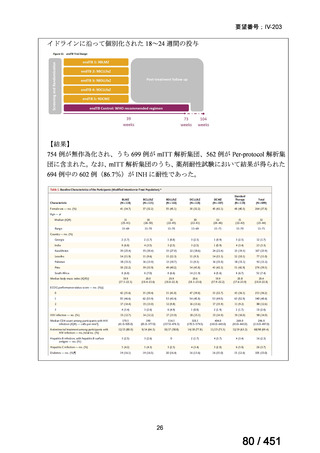
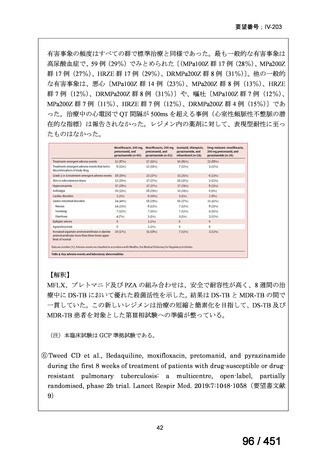
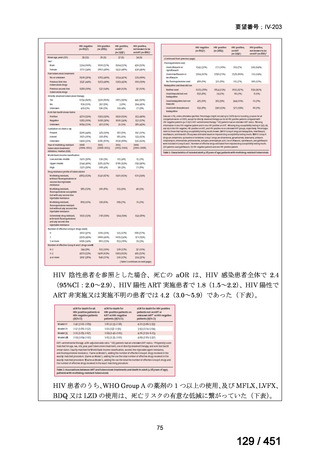
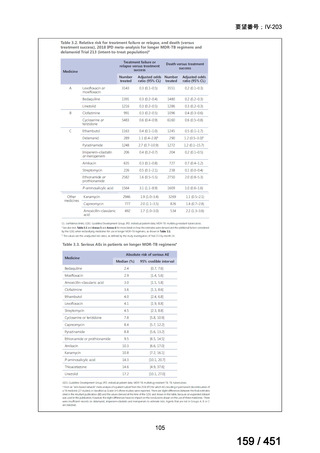
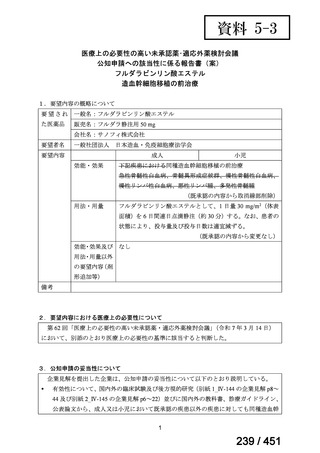
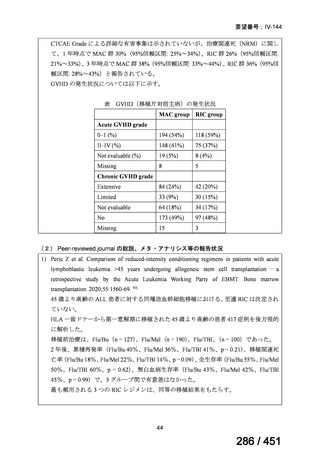
Table 1.3 PICO questions and decisions of the GDG panel(BPaLM 関連記載のみを表示)
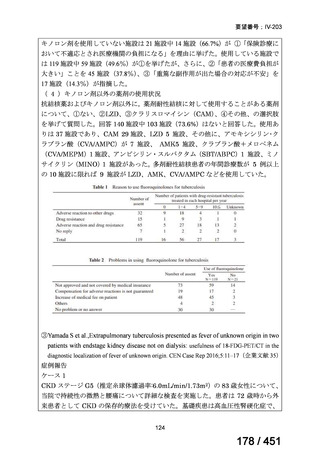
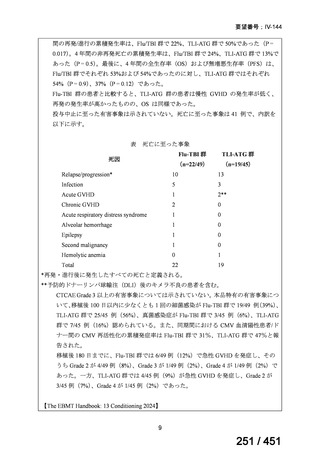
根拠エビデンス:TB-PRACTECAL 試験(推奨の根拠に当該臨床試験のデータが記
載されているが、引用文献としては表示されていない。)
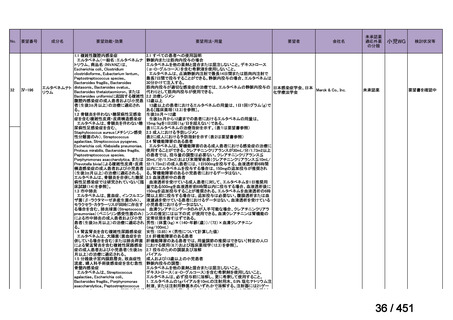
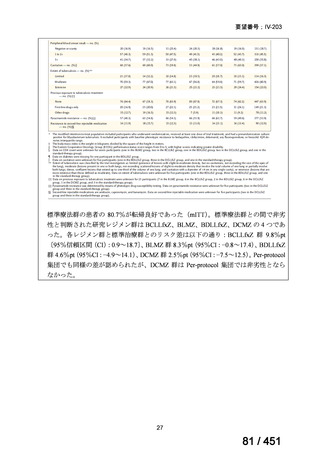
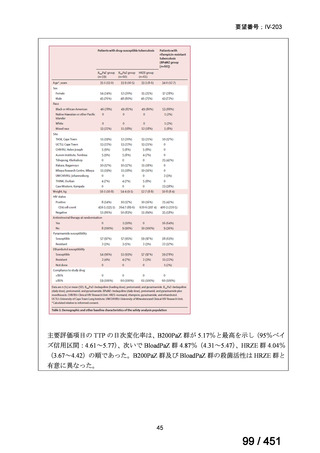
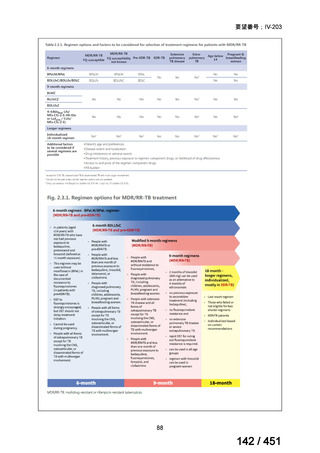
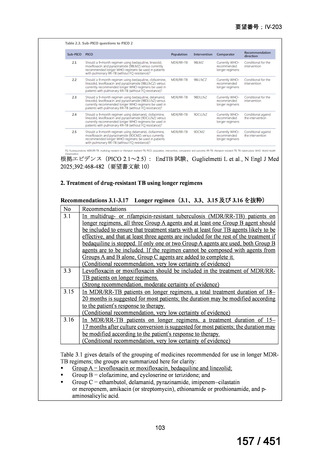
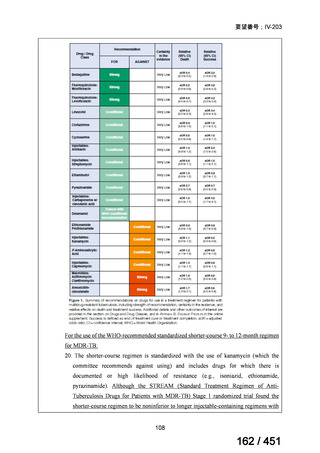
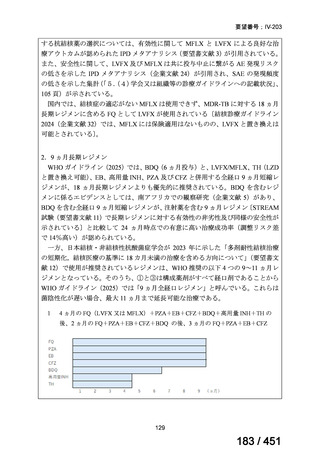
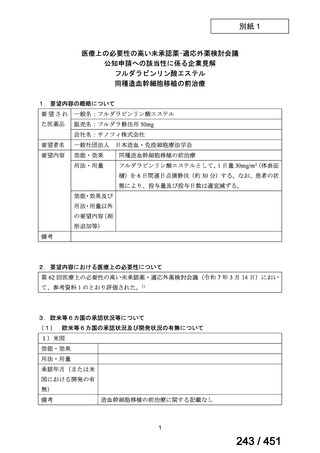
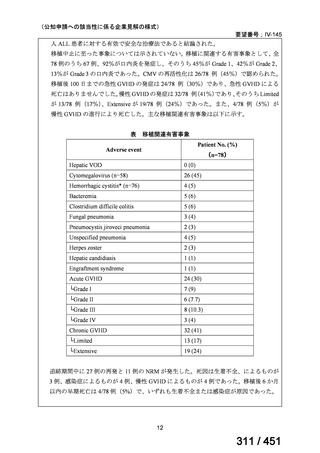
2. Treatment of drug-resistant TB using 9-month regimens
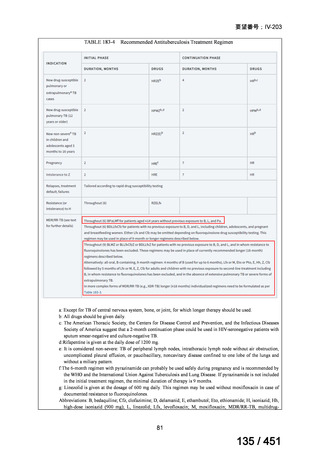
Recommendation 2.1 The 9-month all-oral regimen for MDR/RR-TB
No
2.1
Recommendations
WHO suggests the use of the 9-month all-oral regimen rather than longer
(18-month) regimens in patients with MDR/RR-TB and in whom resistance to
fluoroquinolones has been excluded.
(Conditional recommendation, very low certainty of evidence)
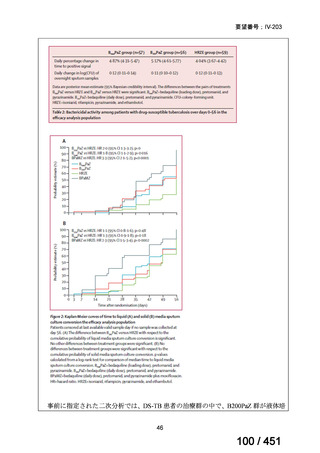
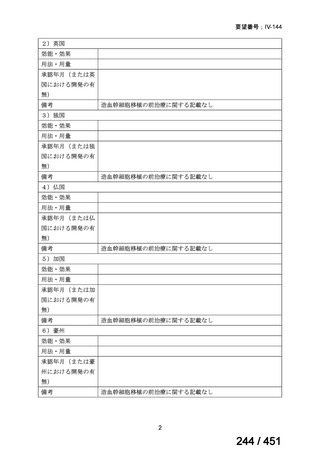
Remarks
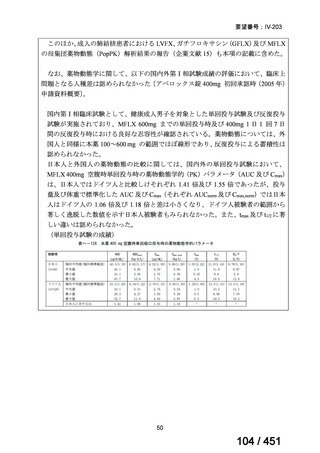
1. The 9-month all-oral regimen consists of bedaquiline (used for 6 months), in combination
with levofloxacin/moxifloxacin, ethionamide, ethambutol, isoniazid (high-dose),
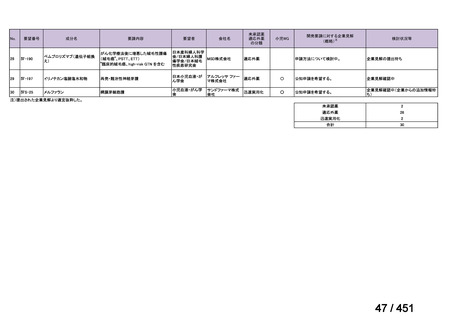
pyrazinamide and clofazimine (for 4 months, with the possibility of extending to 6 months if
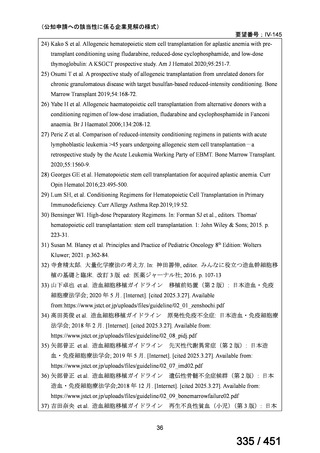
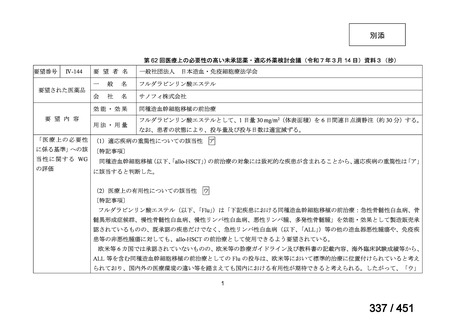
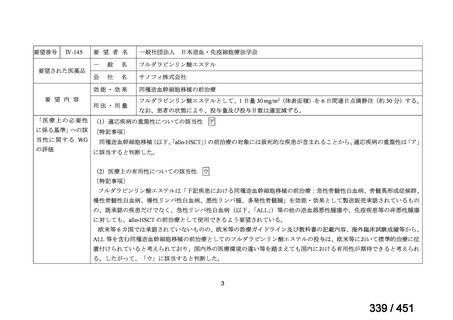
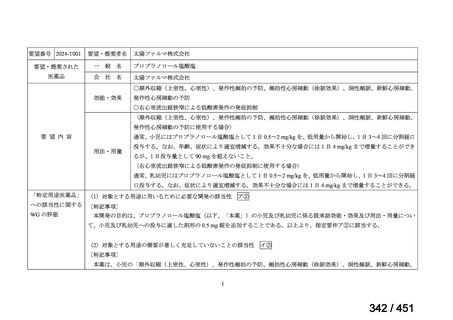
the patient remains sputum smear positive at the end of 4 months), followed by treatment
with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide (for 5 months).
Ethionamide can be replaced by 2 months of linezolid (600 mg daily).
2. A 9-month regimen with linezolid instead of ethionamide may be used in pregnant women,
unlike the regimen with ethionamide.
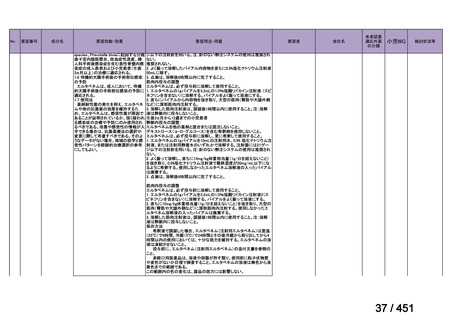
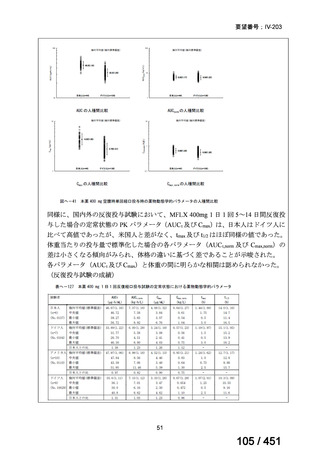
3. This recommendation applies to:
a) people with MDR/RR-TB and without resistance to fluoroquinolones;
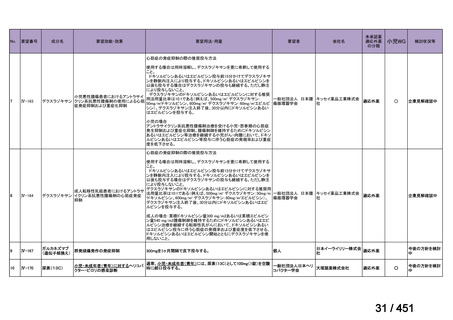
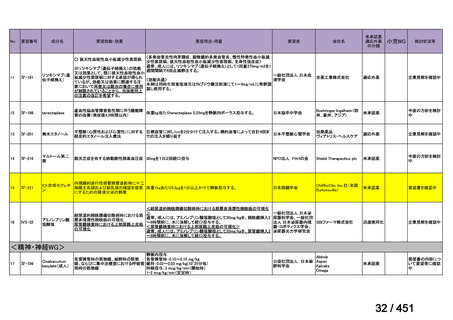
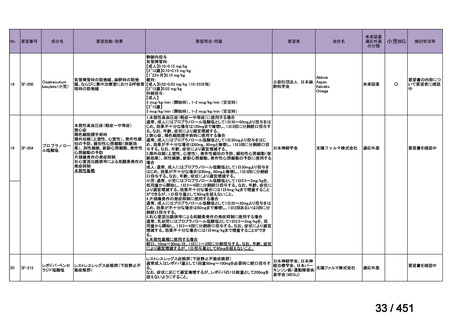
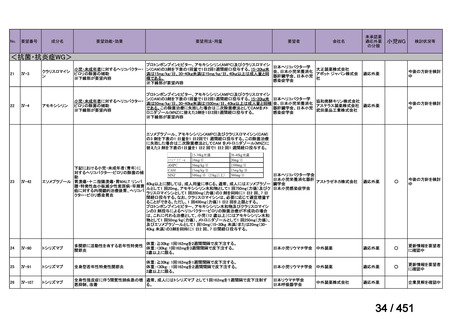
b) patients without extensive TB disease and without severe extrapulmonary TB
c) patients with less than 1 month exposure to bedaquiline, fluoroquinolones, ethionamide,
linezolid and clofazimine; when exposure is greater than 1 month, these patients may
still receive this regimen if resistance to the specific medicines with such exposure has
been ruled out;
d) all people regardless of HIV status;
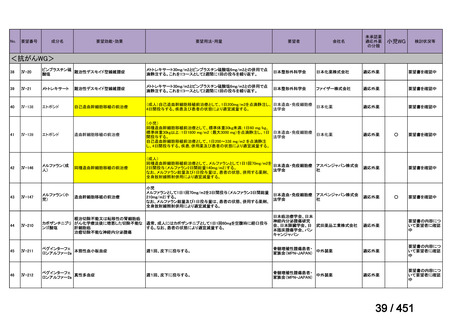
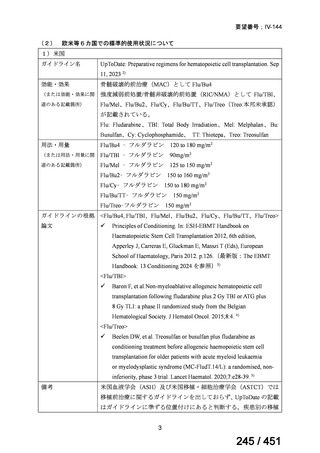
e) children (and patients in other age groups) who do not have bacteriological confirmation
of TB or resistance patterns but who do have a high likelihood of MDR/RR-TB (based
on clinical signs and symptoms of TB, in combination with a history of contact with a
patient with confirmed MDR/RR-TB).
101
155 / 451