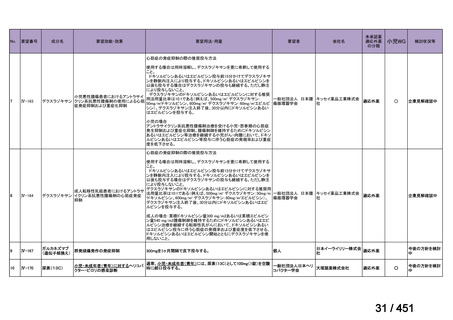
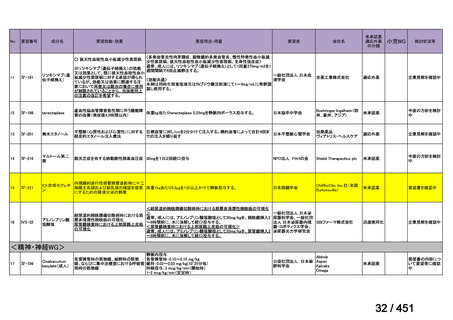
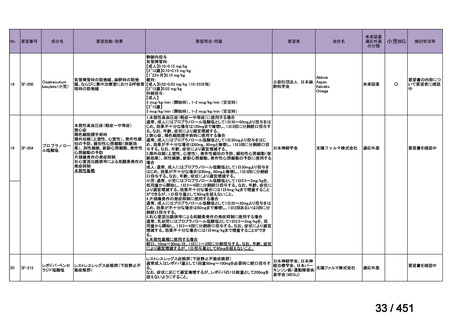
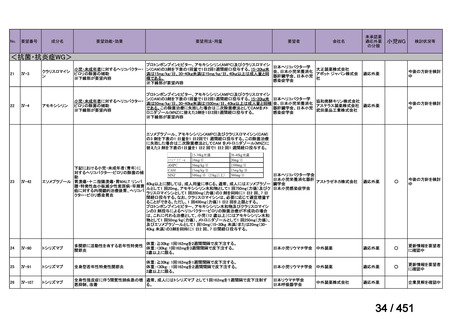
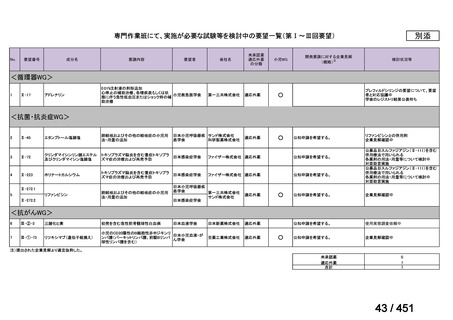
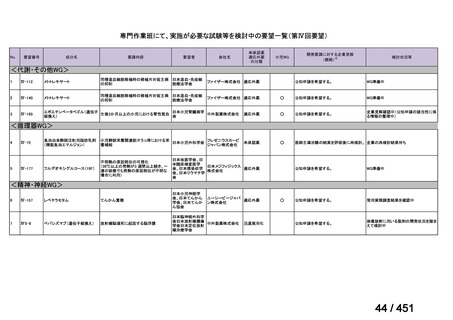
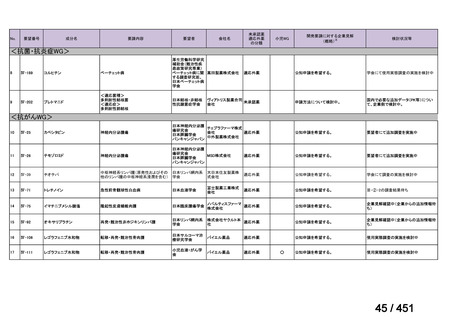
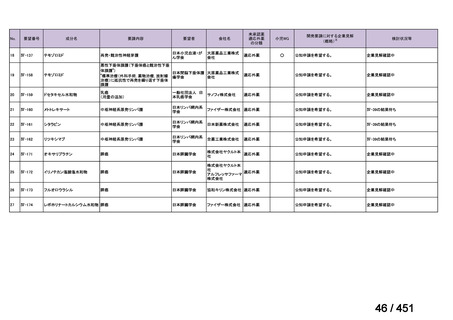
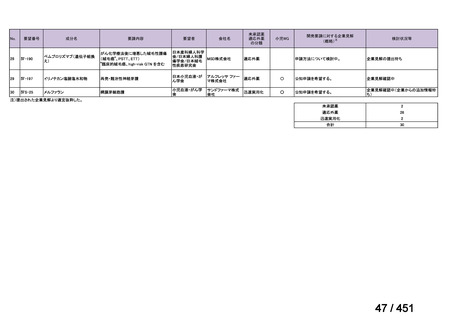
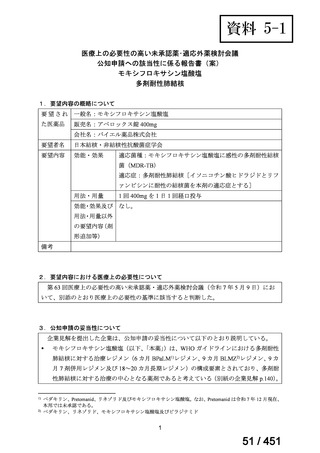
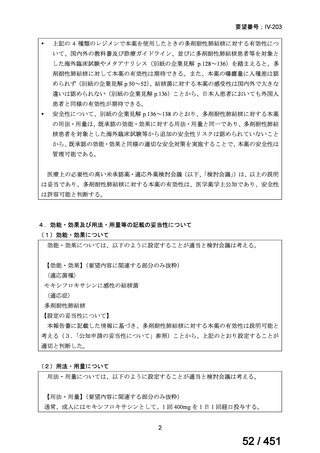
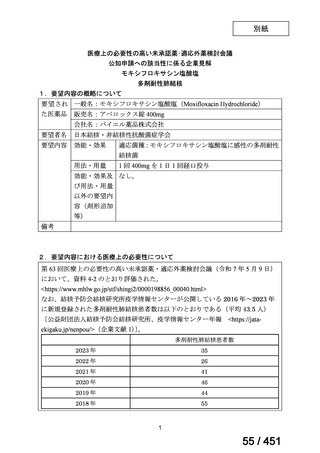
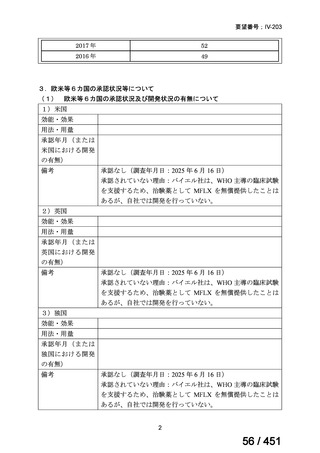
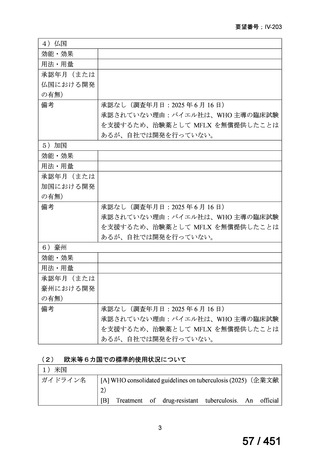
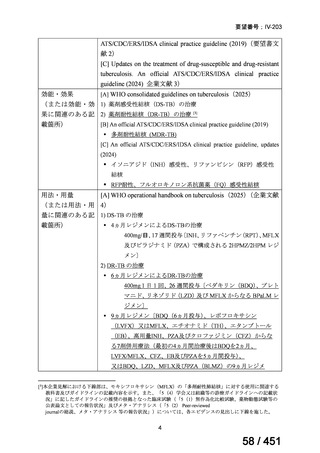
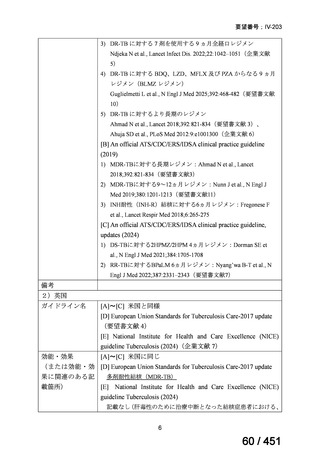
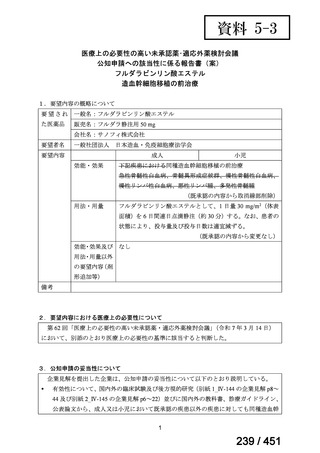
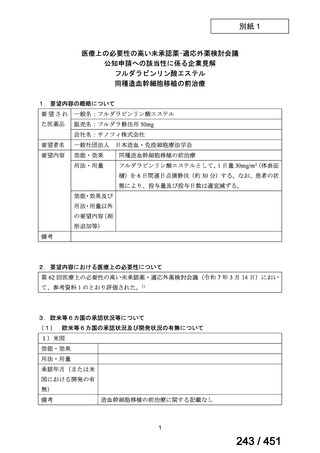
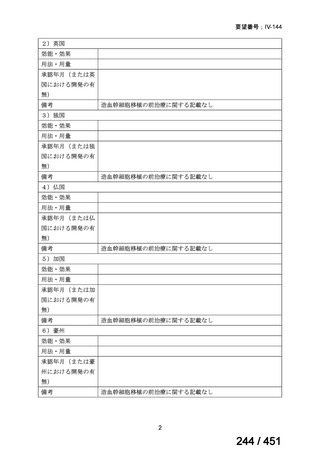
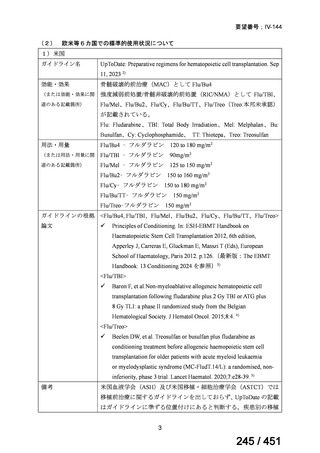
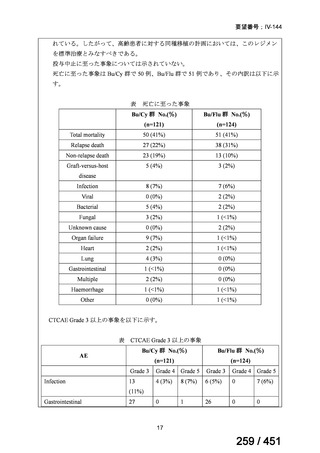
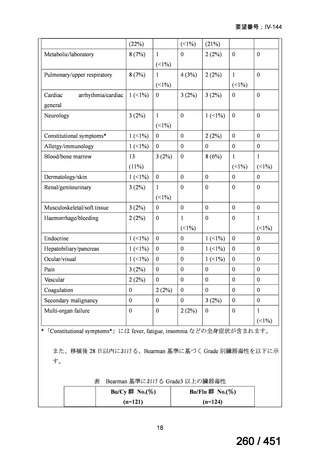
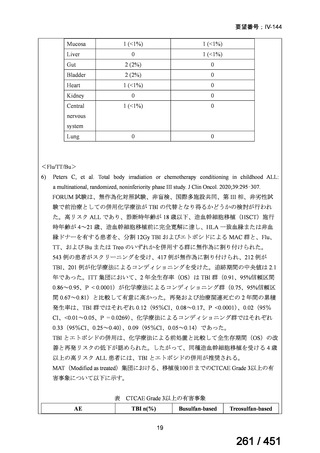
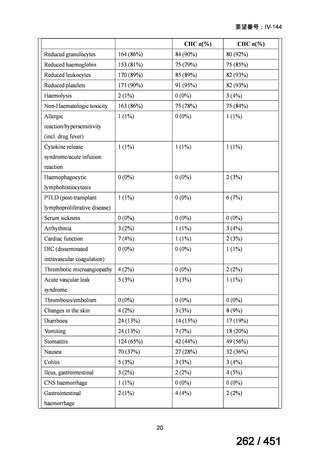
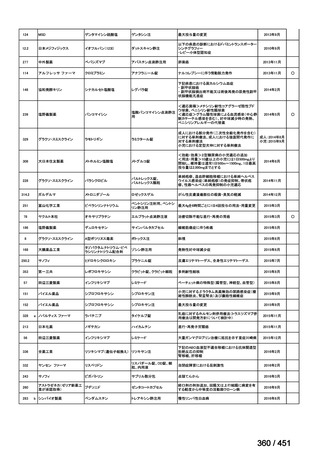
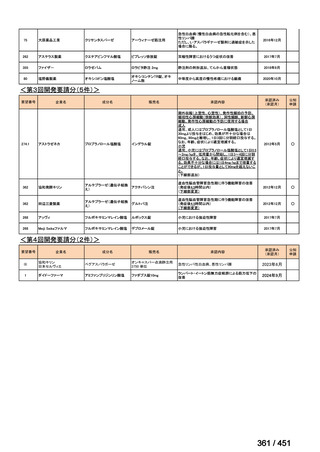
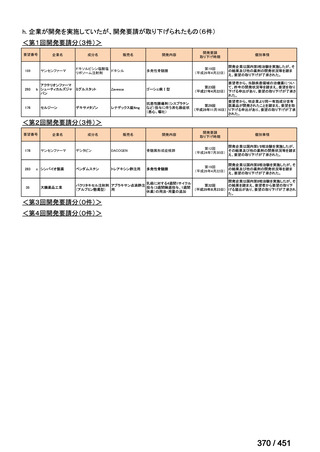
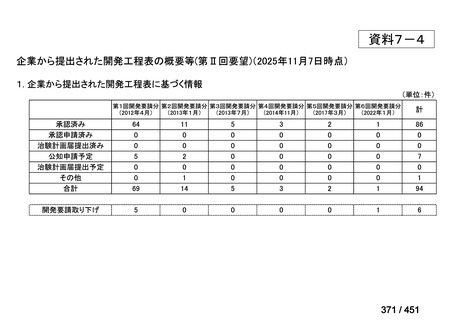
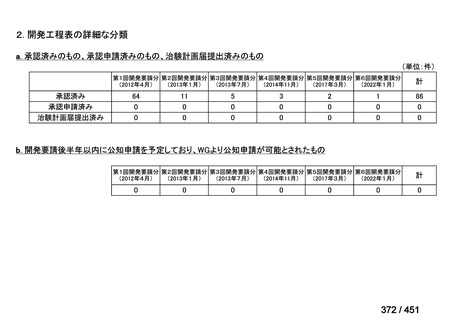
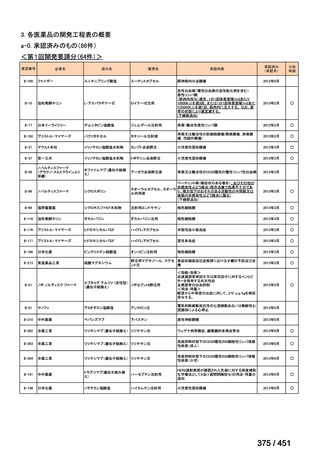
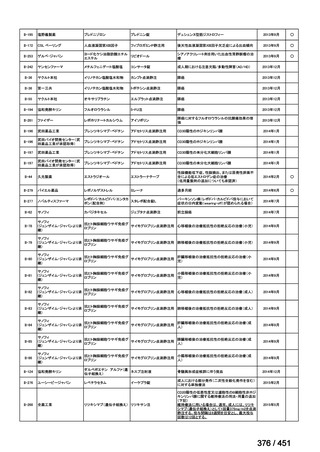
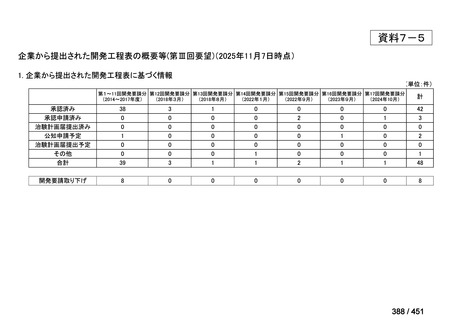
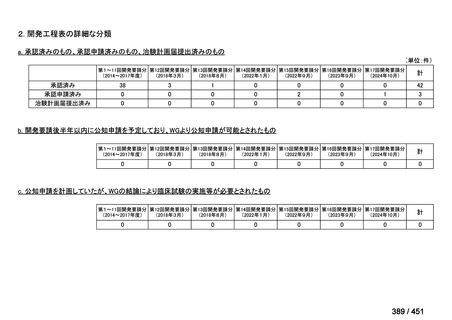
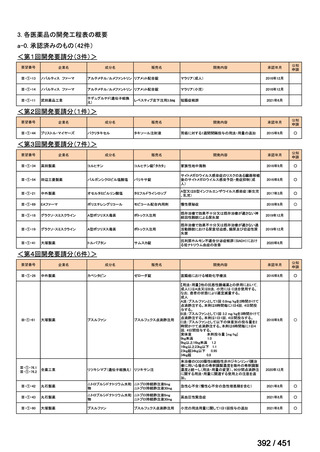
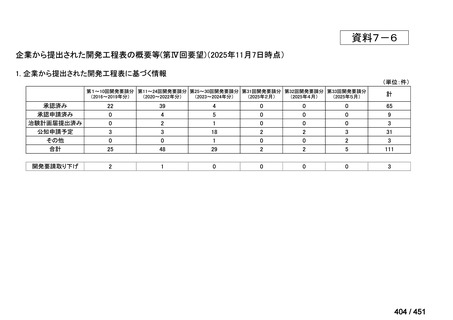
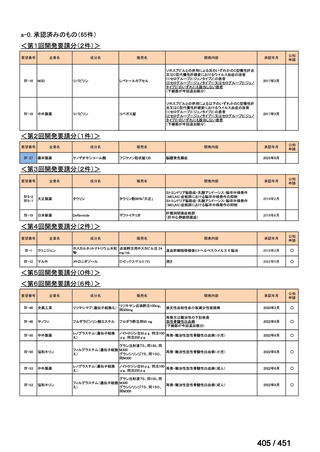
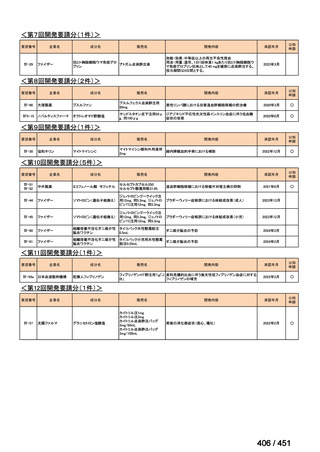
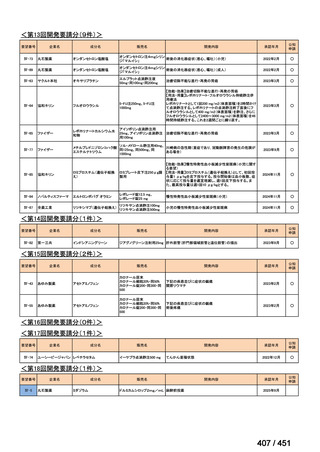
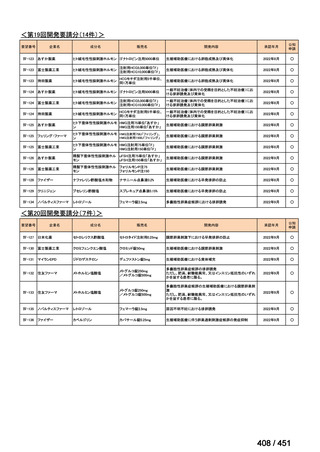
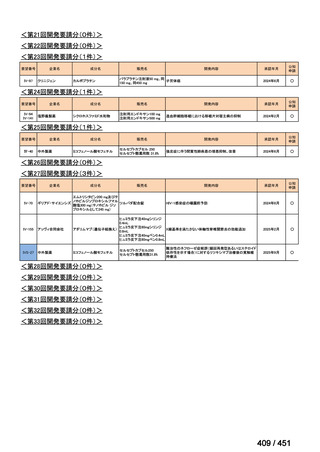
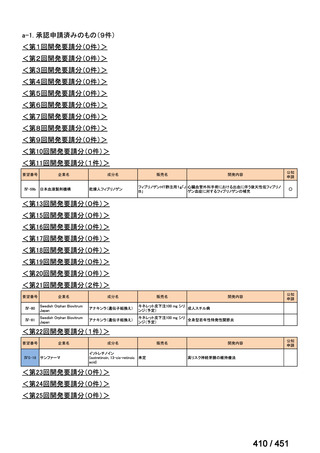
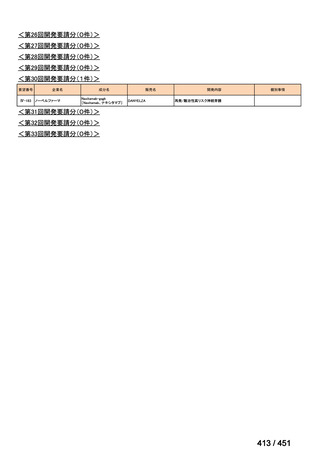
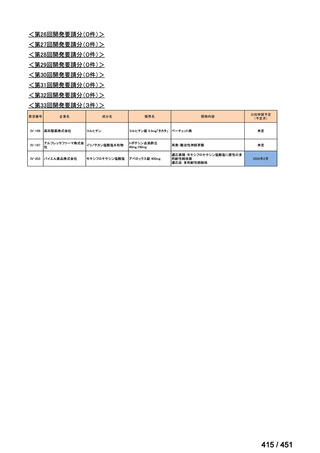
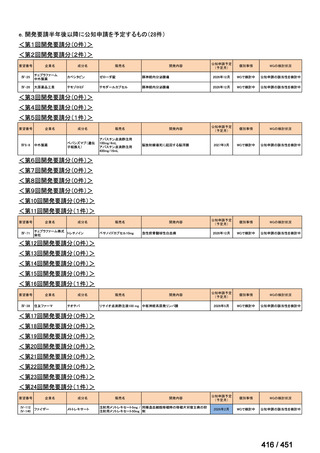
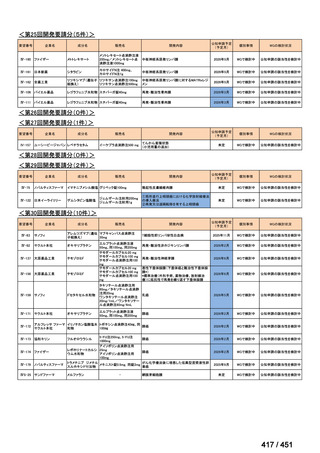
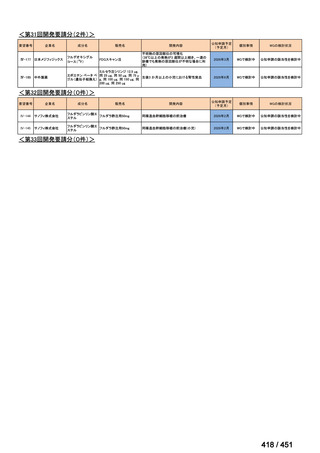
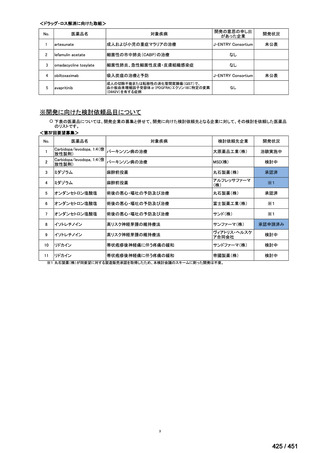
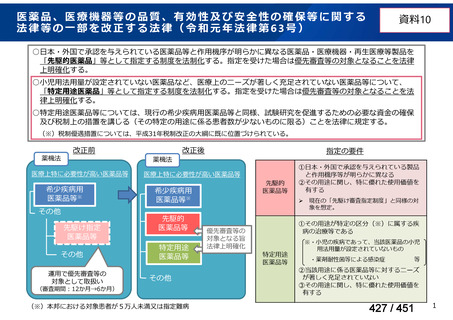
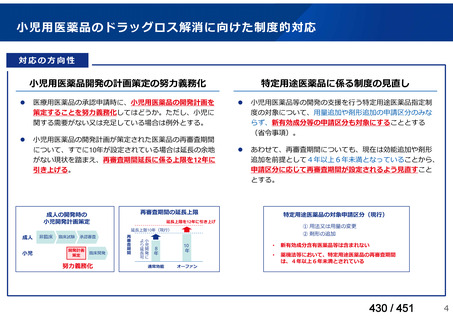
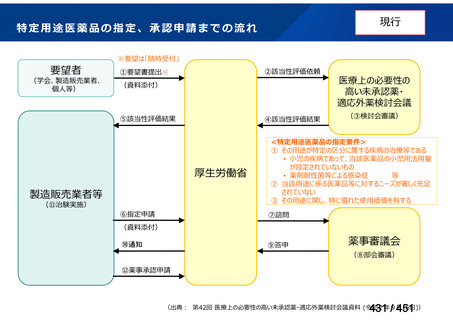
会議資料 (161 ページ)
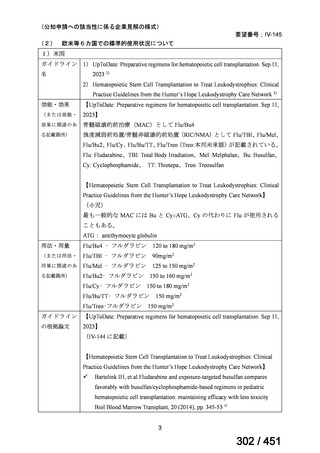
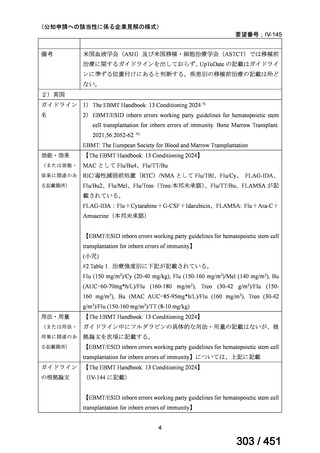
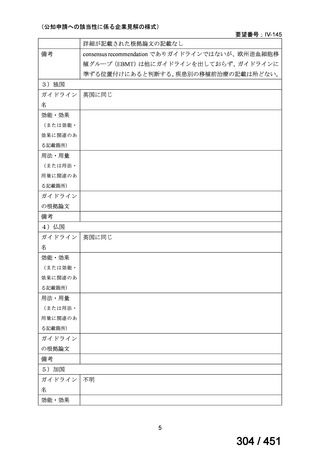
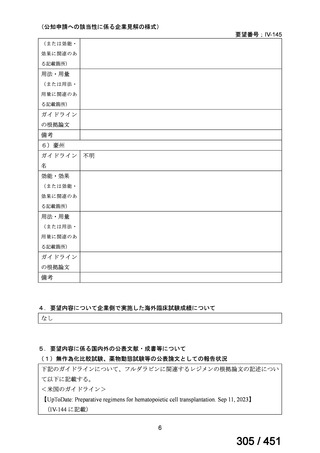
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00044.html |
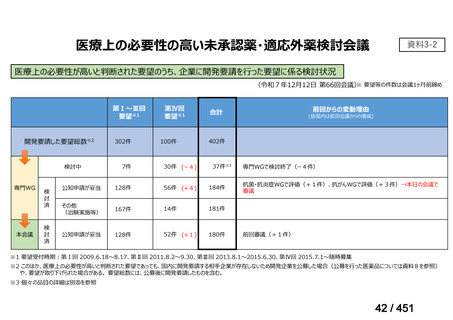
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第66回 12/12)《厚生労働省》 |
ページ画像
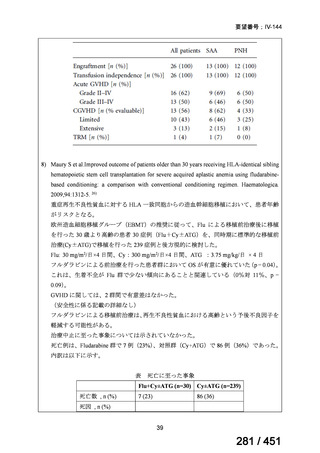
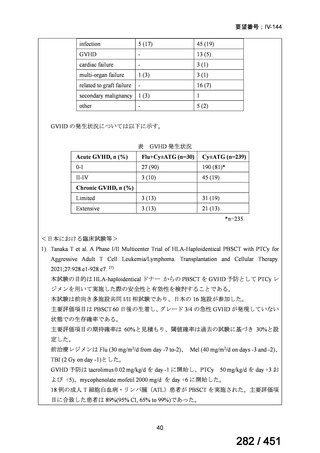
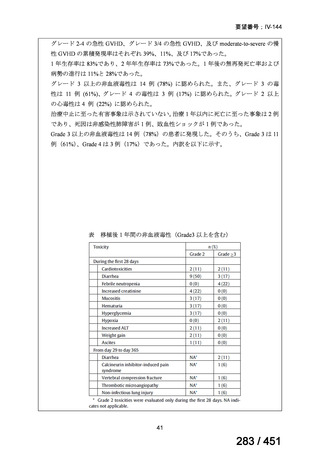
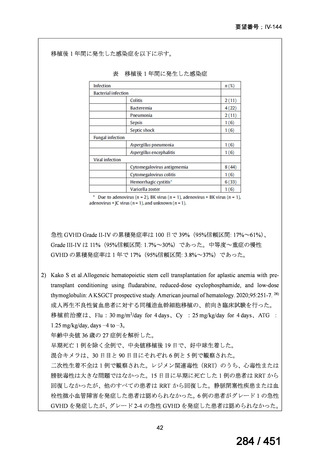
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
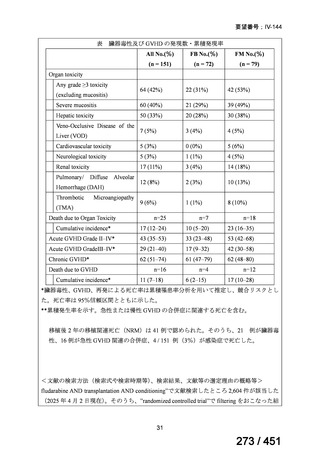
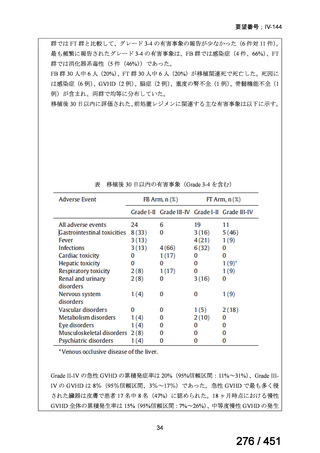
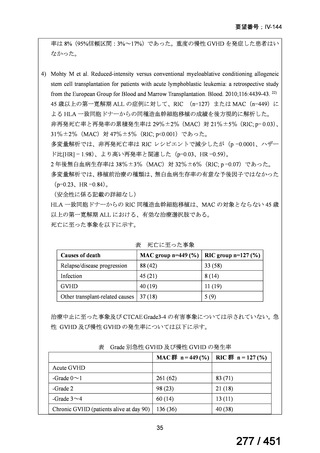
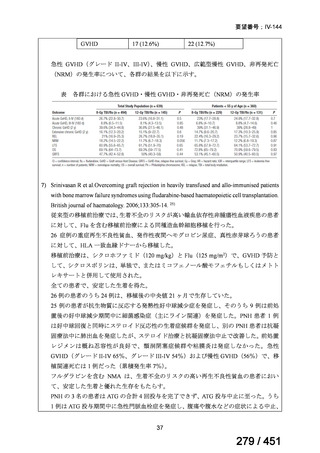
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
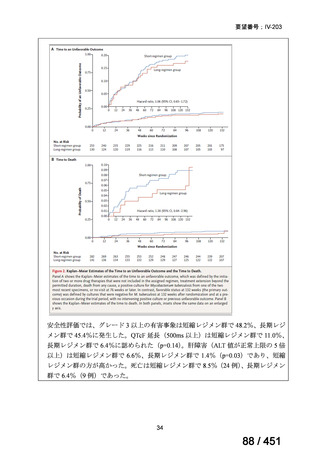
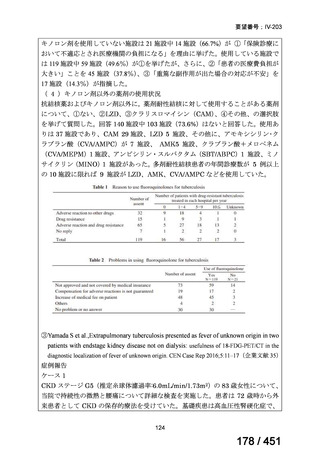
culture conversion?
Recommendation 2: In patients with MDR-TB, we suggest an intensive-phase
duration of treatment of between 5 and 7 months after culture conversion
(conditional recommendation, very low certainty of evidence).
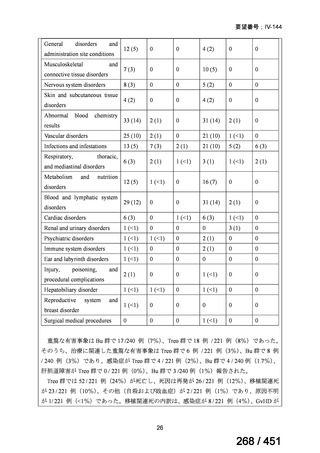
PICO Question 3: Should patients with MDR-TB undergoing continuation-phase
treatment be treated for ≥18 months after culture conversion or <18 months after
culture conversion?
Recommendation 3a: In patients with MDR-TB, we suggest a total treatment
duration of between 15 and 21 months after culture conversion (conditional
recommendations, very low certainty in the evidence).
Recommendation 3b: In patients with pre–XDR-TB and XDR-TB, we suggest a
total treatment duration of between 15 and 24 months after culture conversion
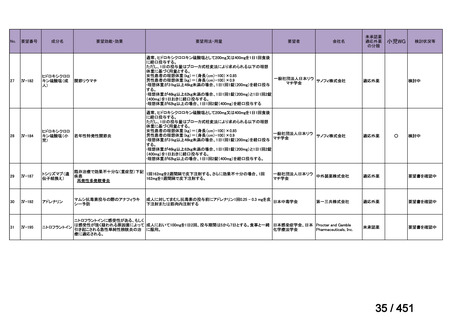
(conditional recommendations, very low certainty in the evidence).
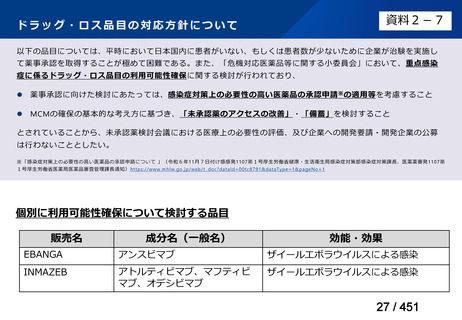
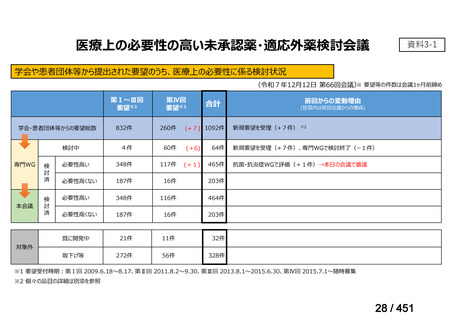
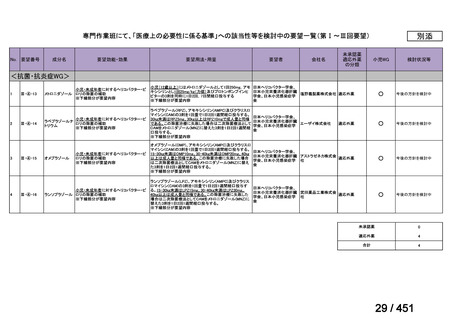
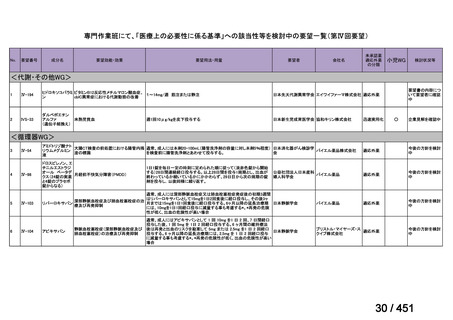
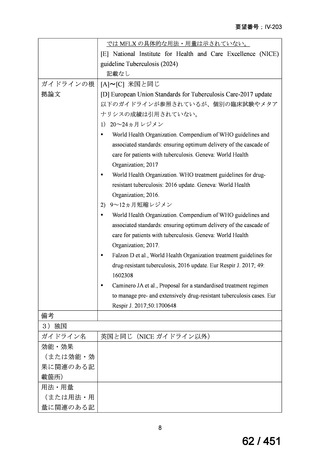
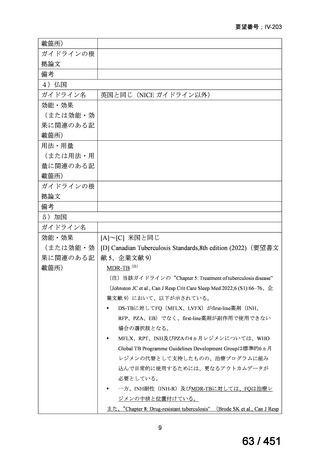
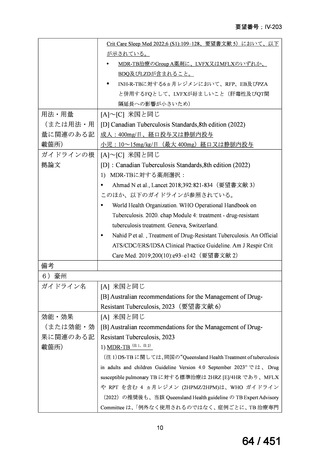
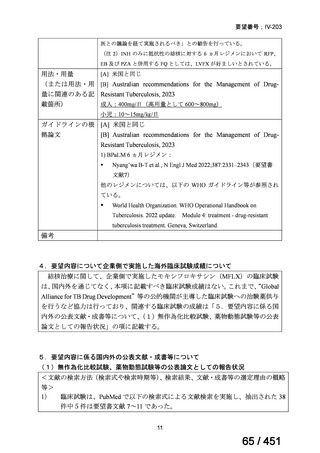
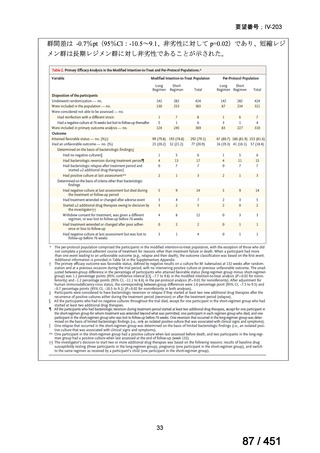
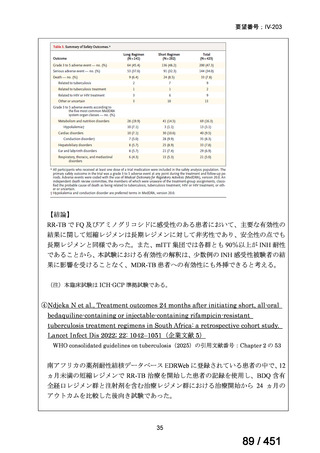
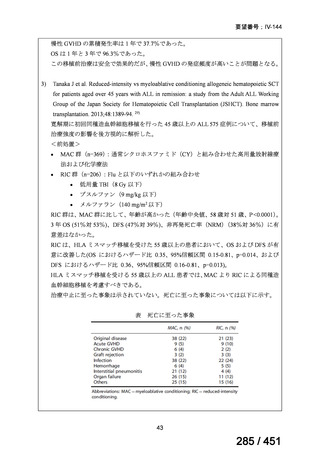
根拠エビデンス:Ahmad N et al., Lancet 2018;392:821-834(要望書文献 3)
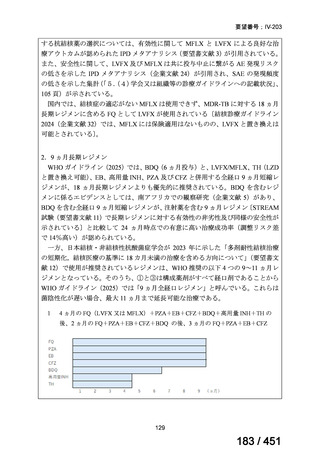
For the selection of oral drugs for MDR-TB treatment (in order of strength of
recommendation):
5. We
recommend
including
a
later-generation
fluoroquinolone
(levofloxacin
or moxifloxacin) (strong recommendation, low certainty of evidence).
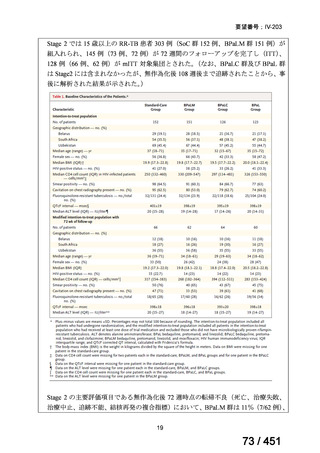
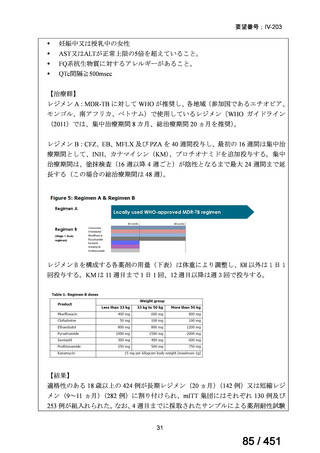
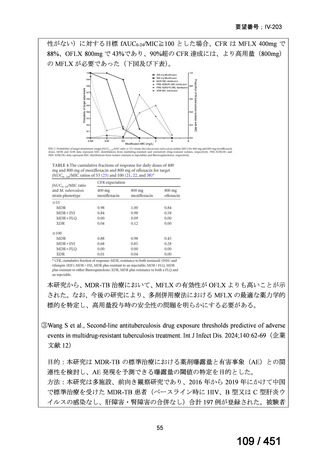
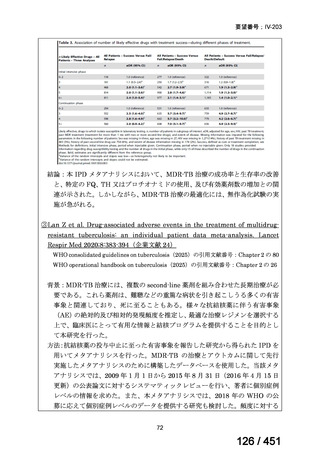
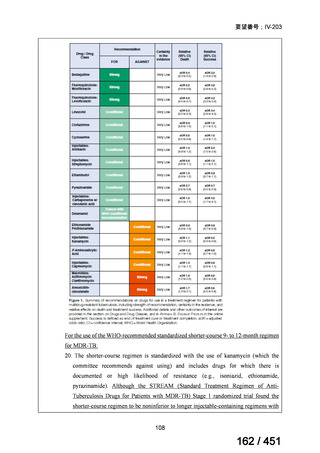
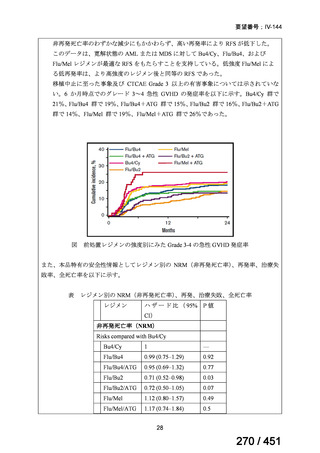
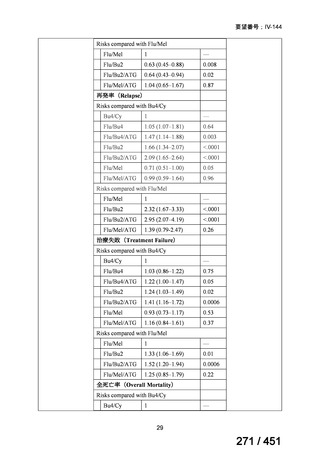
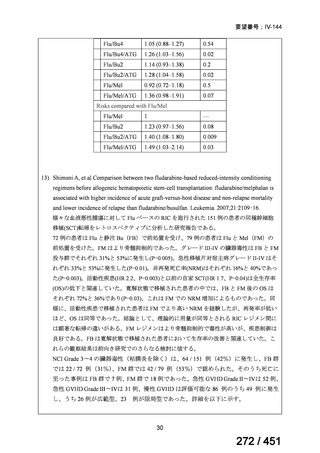
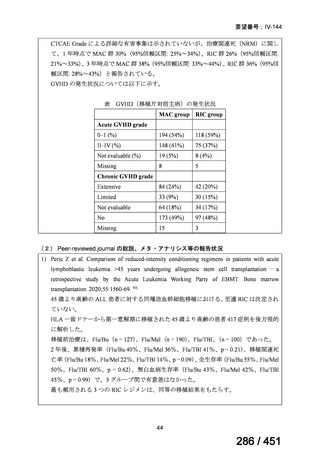
A summary of recommendations on drugs, the certainty in the evidence, and the relative
risks of success and death is provided in Figure 1.
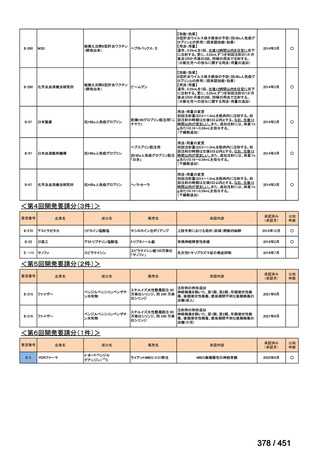
<関連する PICO question>
PICO Question 12: Fluoroquinolones: In patients with MDR-TB, are outcomes
safely improved when regimens include fluoroquinolones compared with regimens
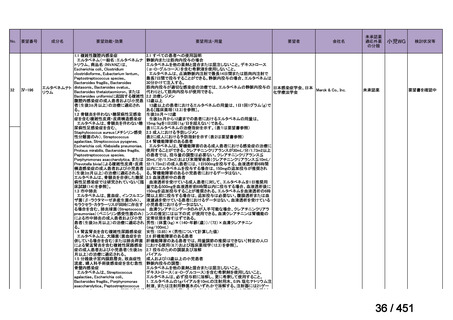
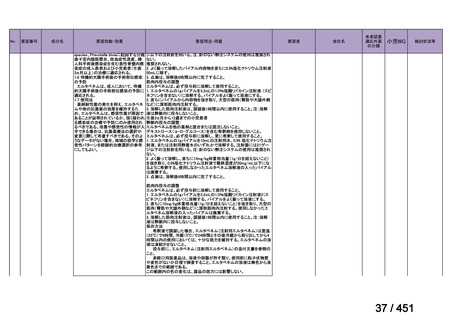
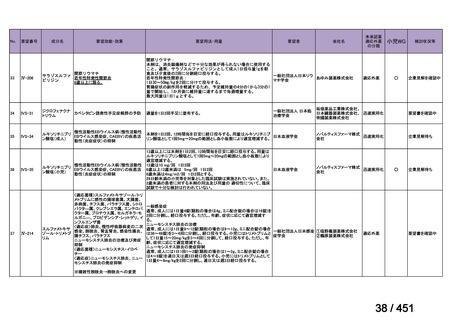
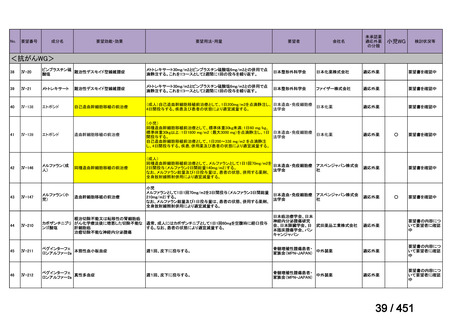
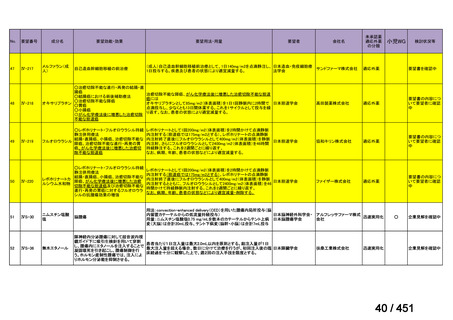
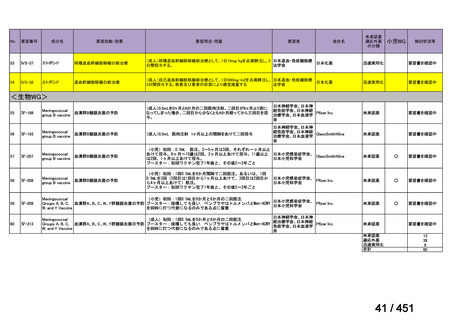
that do not include fluoroquinolones?
Recommendation 12: We recommend including moxifloxacin or levofloxacin in a
regimen for treatment of patients with MDR-TB (strong recommendation, low
certainty in the evidence). Our recommendation for the use of moxifloxacin or
levofloxacin is strong despite very low certainty in the evidence because we viewed
the significant reduction in mortality, improved treatment success, and relatively
few adverse effects associated with MDR-TB treatment that includes these latergeneration fluoroquinolones (compared with no fluoroquinolones) as having a
particularly favorable balance of benefits over harms.
根拠エビデンス:Ahmad N et al., Lancet 2018;392:821-834(要望書文献 3)
107
161 / 451