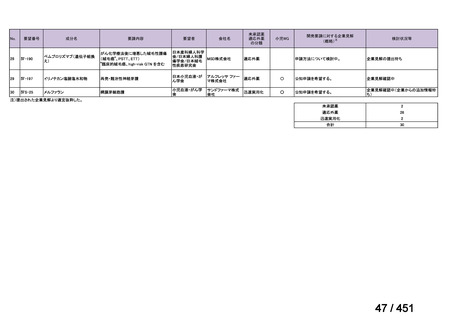
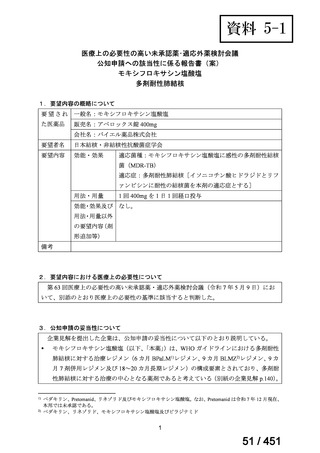
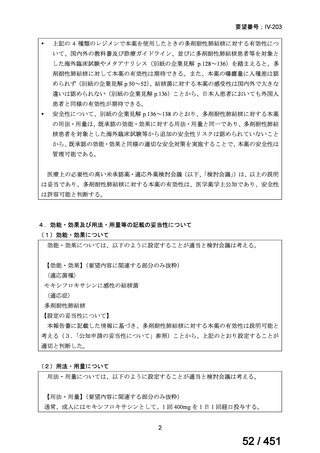
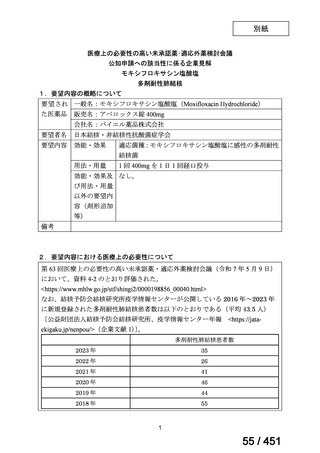
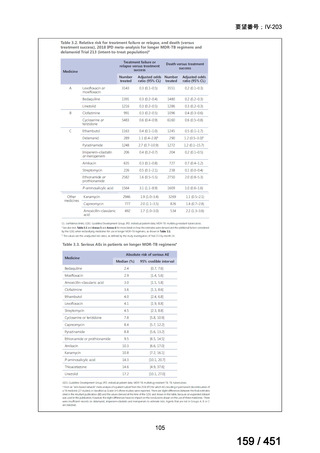
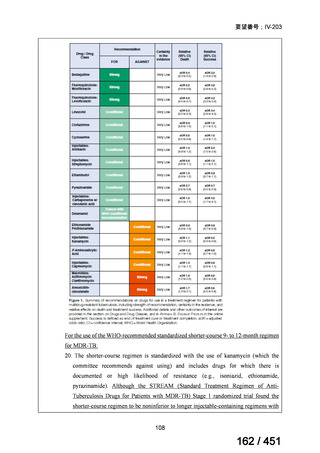
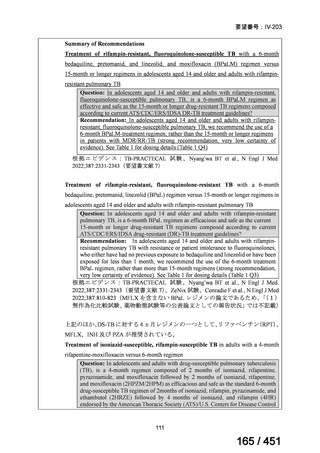
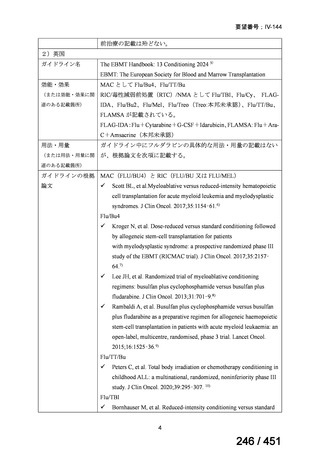
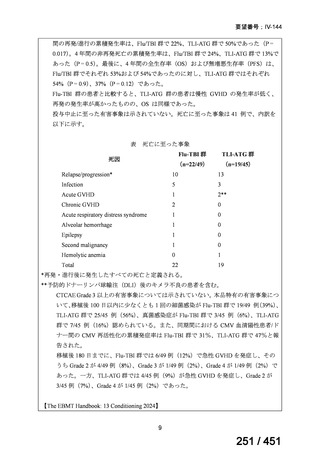
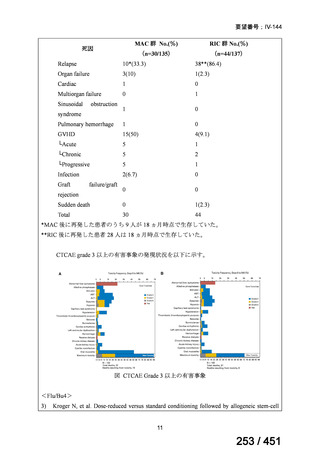
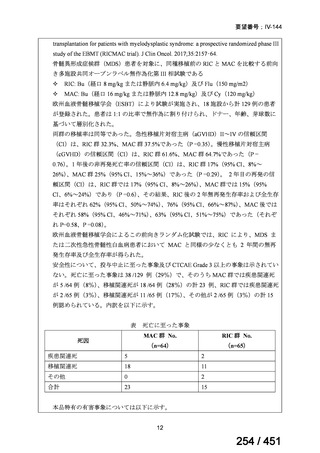
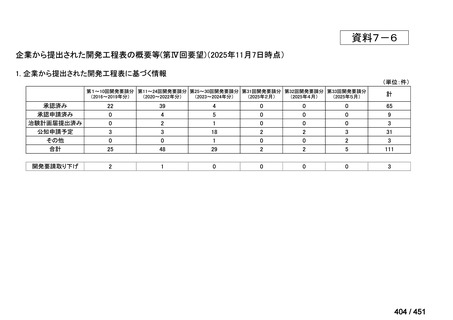
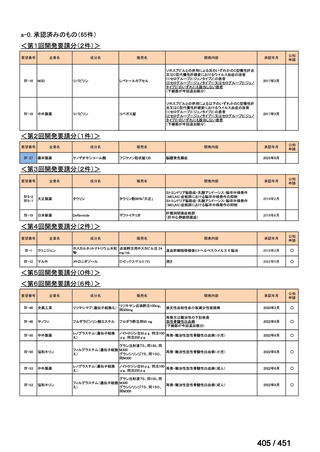
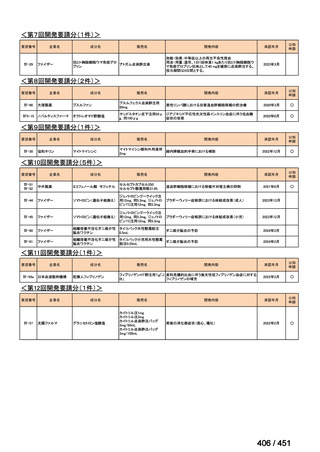
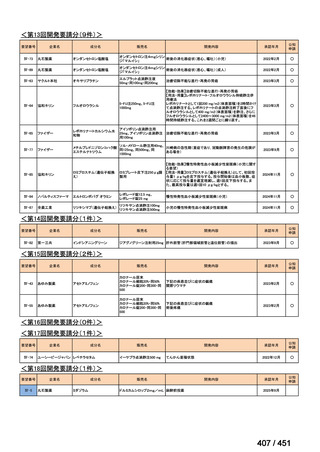
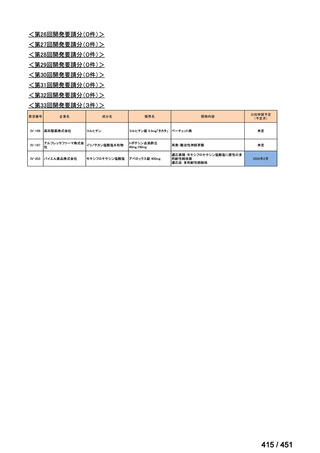
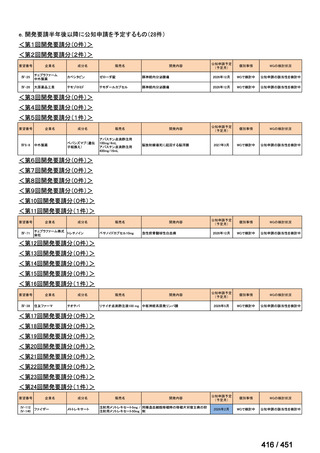
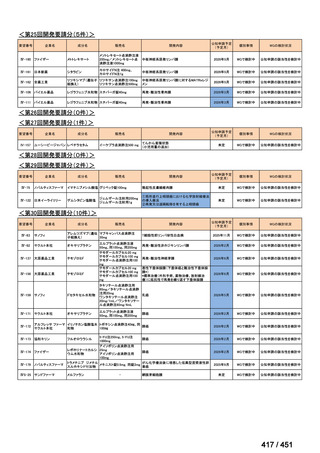
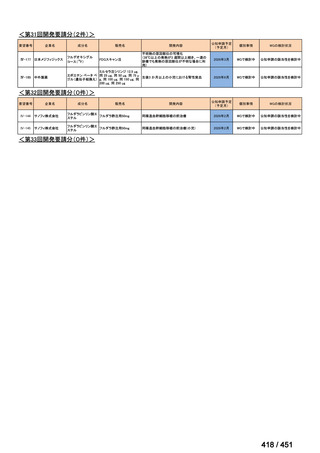
会議資料 (163 ページ)
出典
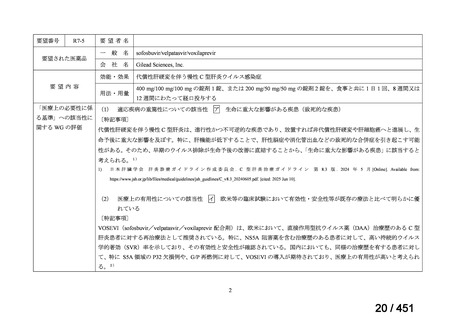
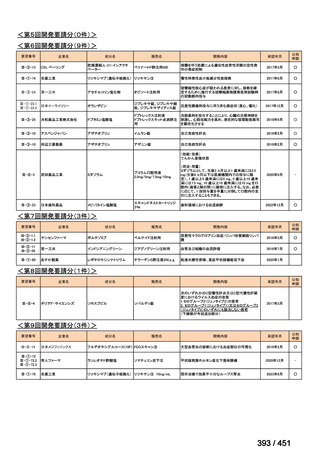
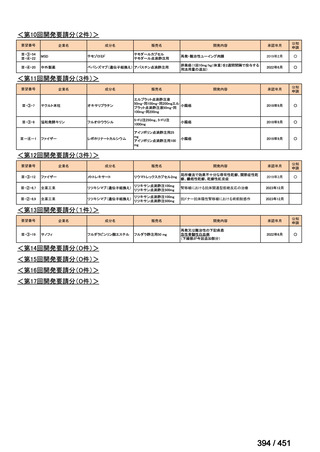
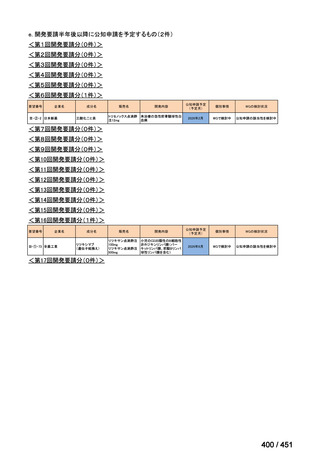
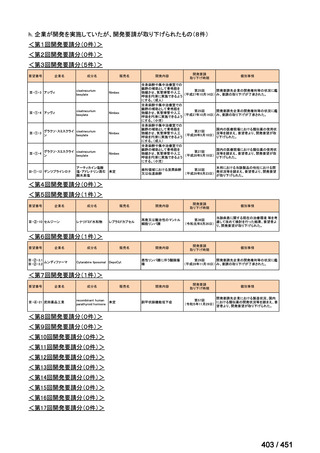
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00044.html |
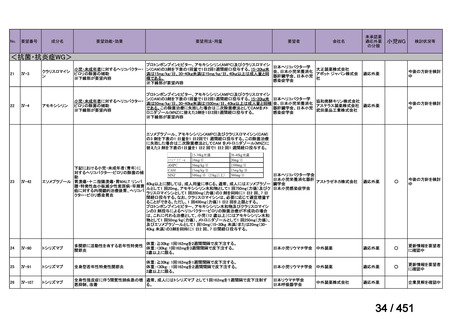
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第66回 12/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
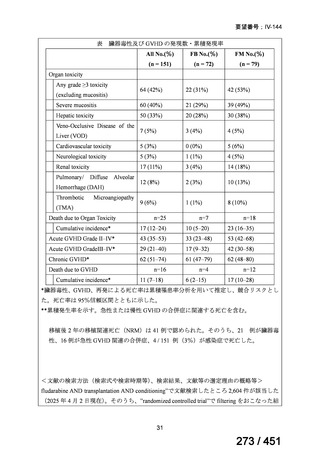
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
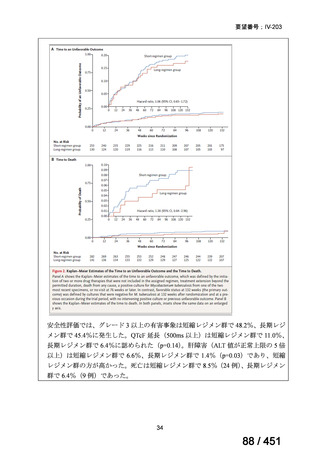
respect to the primary efficacy outcome (Nunn AJ et al., N Engl J Med 2019;380:12011213), the guideline committee cannot make a recommendation either for or against this
standardized shorter-course regimen, compared with longer individualized all-oral
regimens that can be composed in accordance with the recommendations in this practice
guideline. We make a research recommendation for the conduct of randomized clinical
trials evaluating the efficacy, safety, and tolerability of modified shorter-course
regimens that include newer oral agents, exclude injectables, and include drugs for
which susceptibility is documented or highly likely.
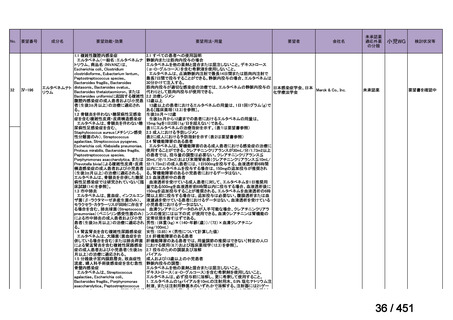
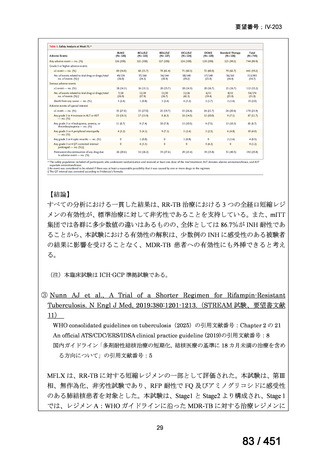
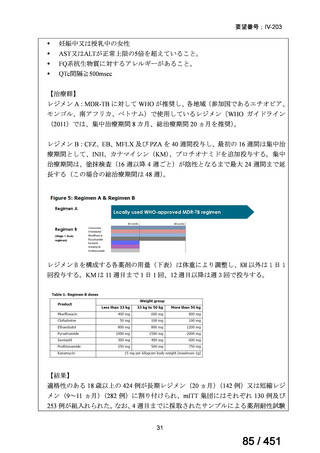
<関連する PICO question>
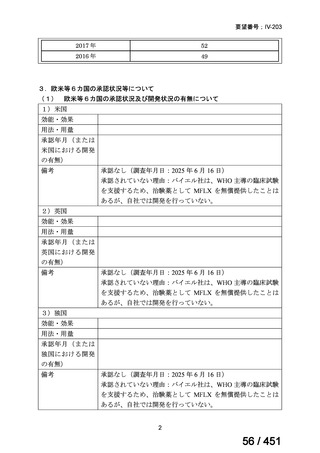
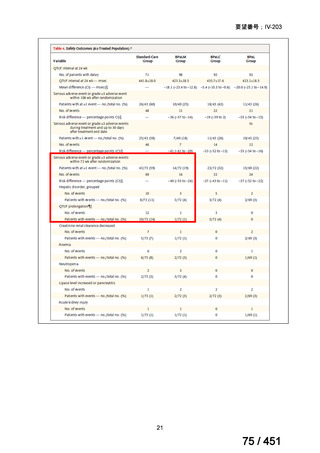
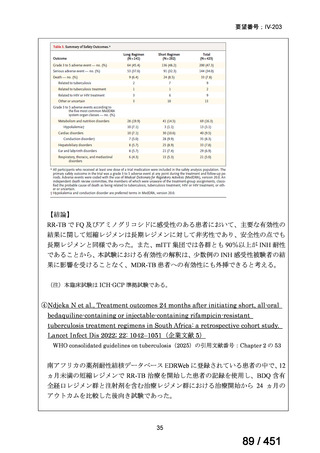
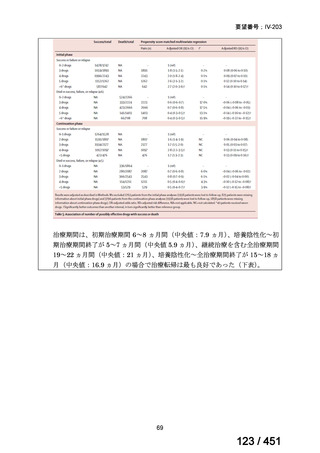
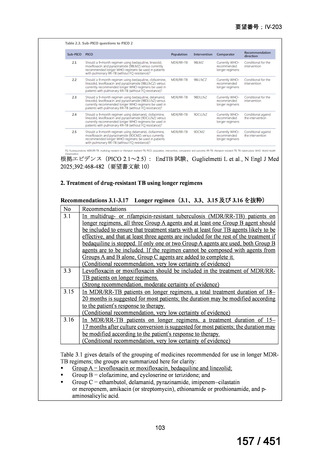
PICO Question 18: Shorter-course, standardized regimen: In patients with MDRTB, does treatment with a standardized MDR-TB regimen for ≤12 months lead to
better outcomes than treatment with an MDR-TB regimen for 18–24 months?
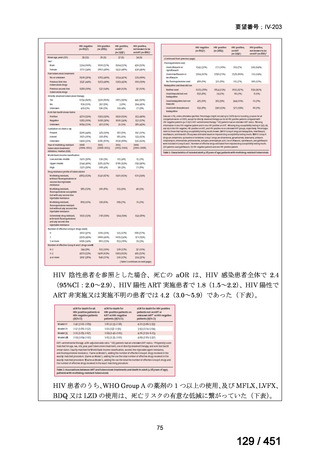
Recommendation 18: The shorter-course regimen is standardized with the use of
kanamycin (which the committee recommends against using) and includes drugs
for which there is documented or high likelihood of resistance (e.g., isoniazid,
ethionamide, pyrazinamide). Although the STREAM Stage 1 randomized trial
found the shorter-course regimen to be noninferior to longer injectable-containing
regimens with respect to the primary efficacy outcome (Nunn AJ et al., N Engl J
Med 2019;380:1201-1213), the guideline committee cannot make a
recommendation either for or against this standardized shorter-course regimen,
compared with longer individualized all-oral regimens that can be composed in
accordance with the recommendations in this practice guideline. We make a
research recommendation for the conduct of randomized clinical trials evaluating
the efficacy, safety, and tolerability of modified shorter-course regimens that
include newer oral agents, exclude injectables, and include drugs for which
susceptibility is documented or highly likely.
根拠エビデンス:STREAM 試験、Nunn AJ et al., N Engl J Med 2019;380:1201-1213(要
望書文献 11)
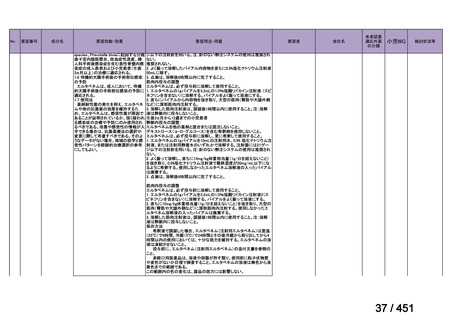
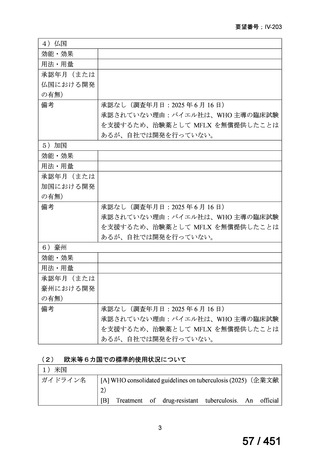
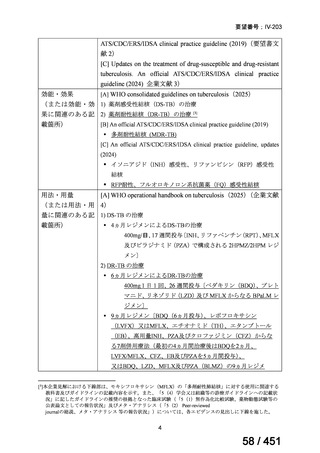
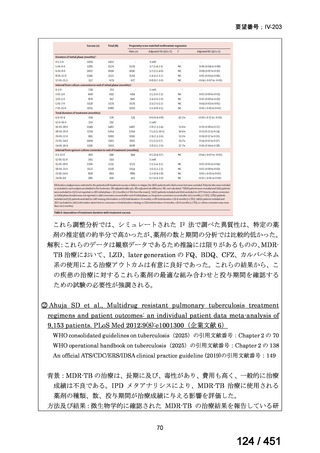
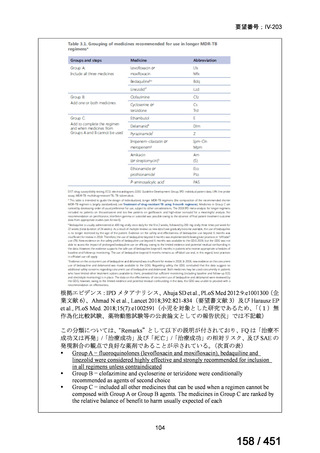
For the treatment of isoniazid-resistant TB:
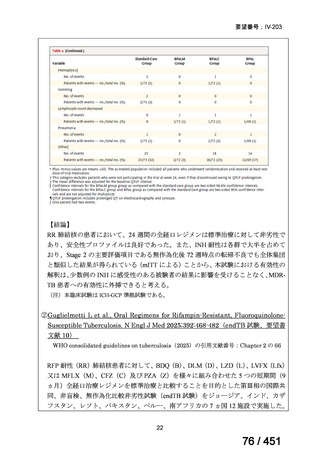
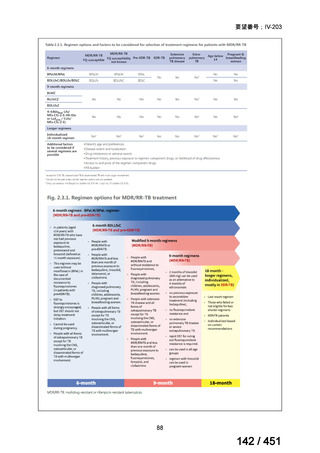
23. We suggest adding a later-generation fluoroquinolone to a 6-month regimen of daily
rifampin, ethambutol, and pyrazinamide for patients with isoniazid-resistant TB
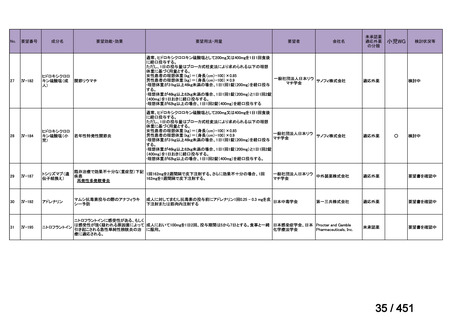
(conditional recommendation, very low certainty in the evidence).
24. In patients with isoniazid-resistant TB treated with a daily regimen of a later-generation
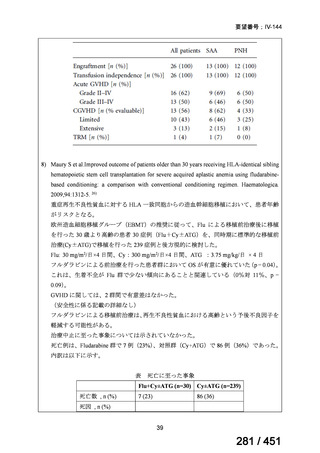
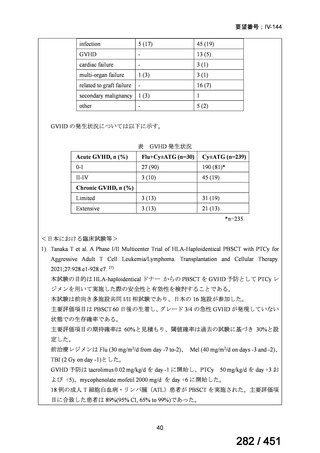
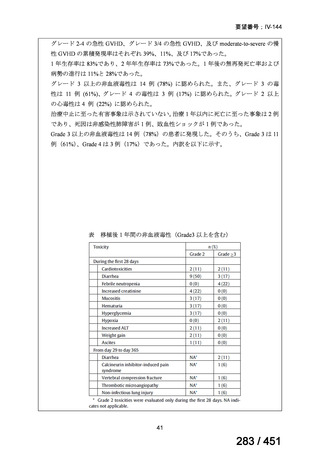
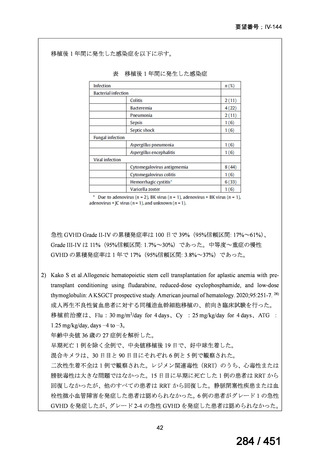
fluoroquinolone, rifampin, ethambutol, and pyrazinamide, we suggest that the duration
of pyrazinamide can be shortened to 2 months in selected situations (i.e., noncavitary
and lower burden disease or toxicity from pyrazinamide) (conditional recommendation,
very low certainty in the evidence).
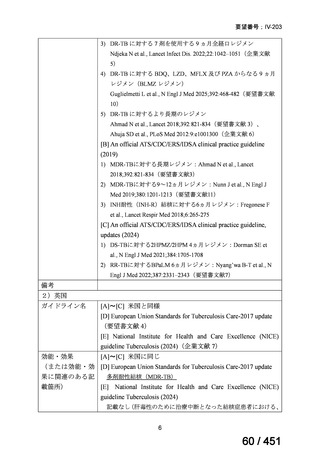
<関連する PICO question>
PICO Question 20—Treatment of isoniazid-resistant TB: PICO Question 20a:
Should patients with isoniazid-resistant TB be treated with a regimen composed of
a fluoroquinolone, rifampin, ethambutol, and pyrazinamide for 6 months compared
with rifampin, ethambutol, and pyrazinamide (without a fluoroquinolone) for
6 months?
109
163 / 451