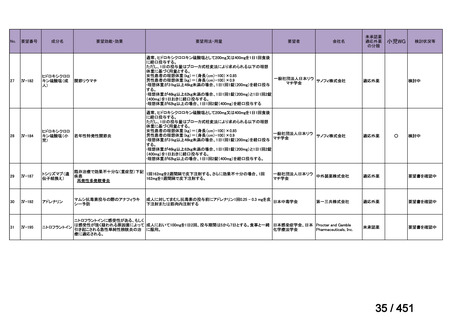
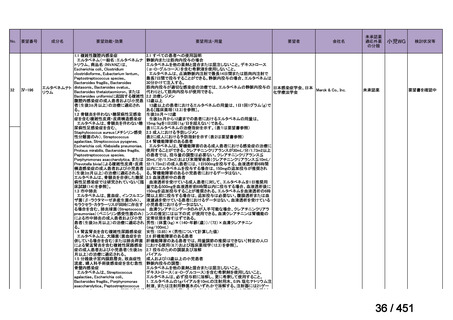
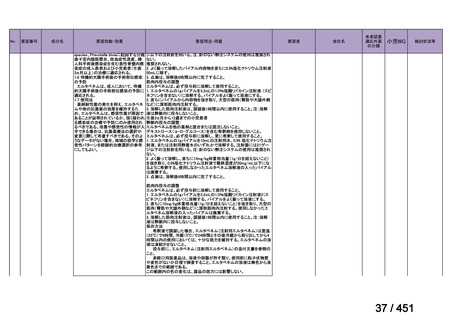
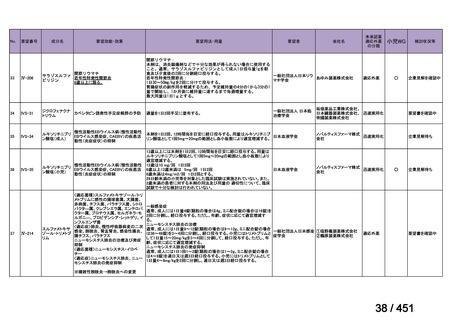
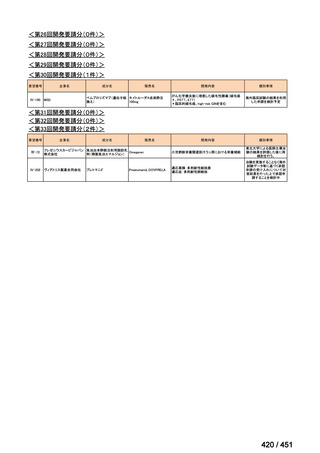
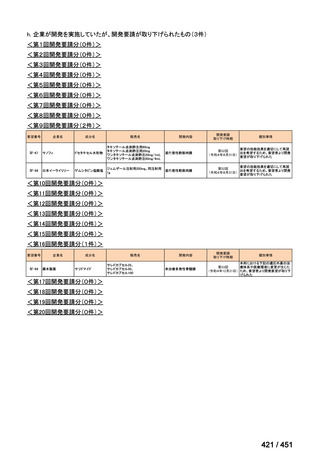
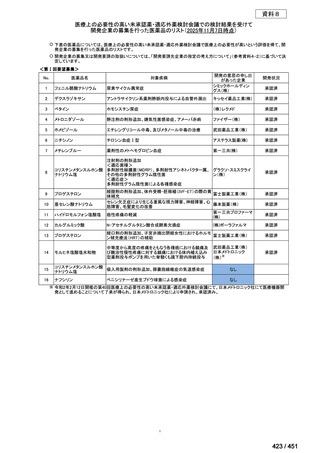
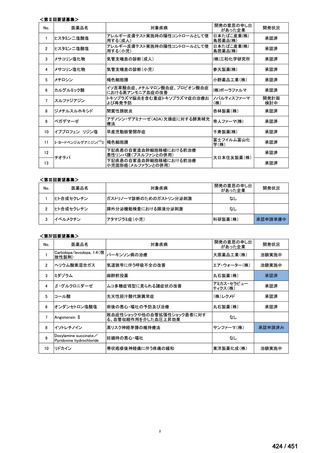
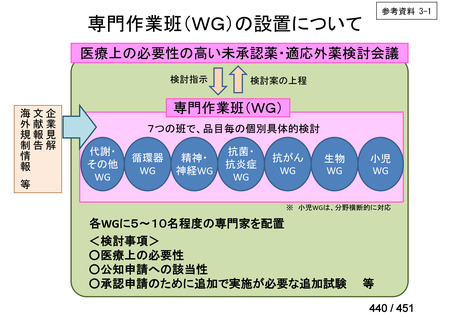
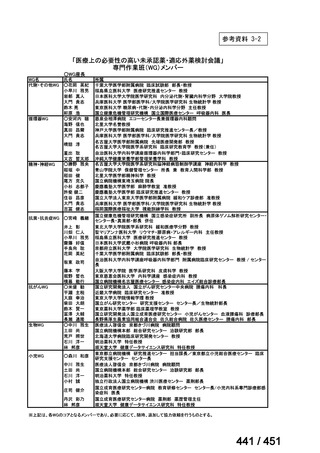
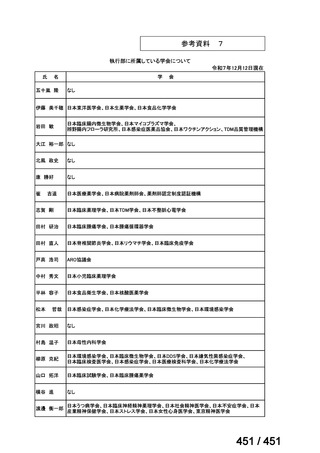
会議資料 (168 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00044.html |
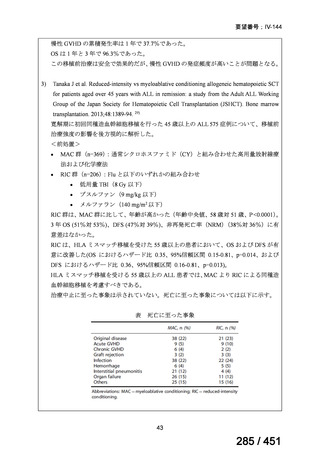
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第66回 12/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
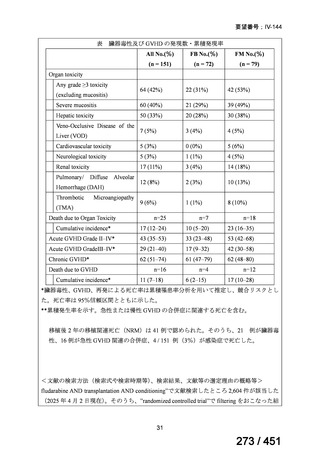
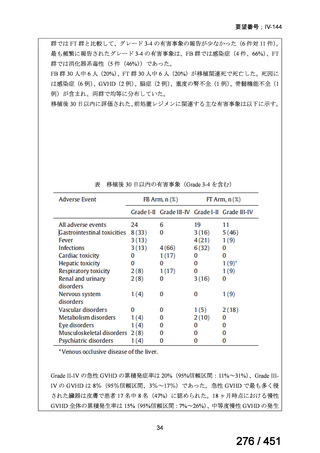
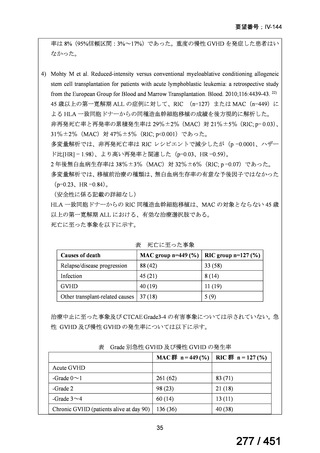
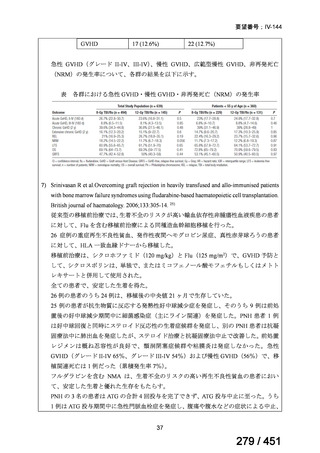
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
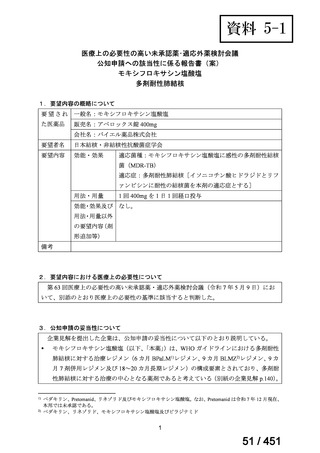
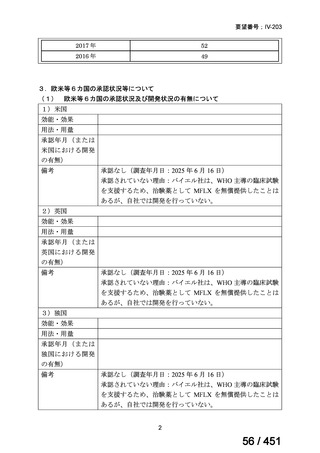
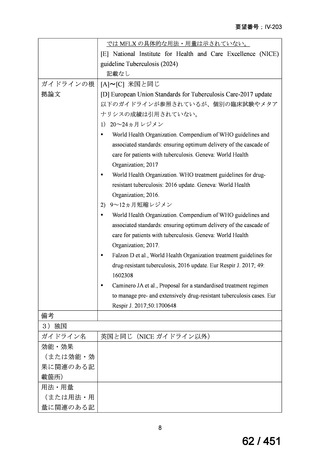
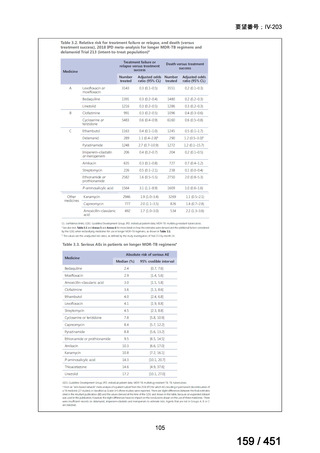
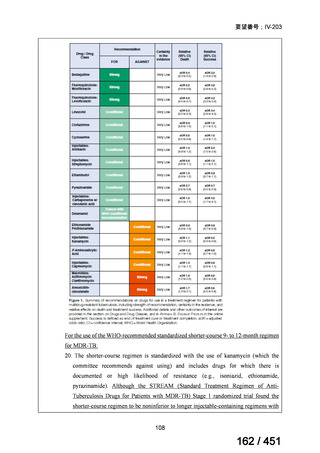
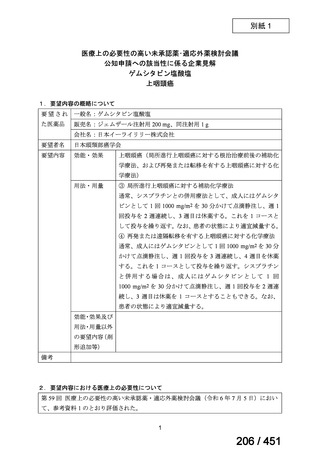
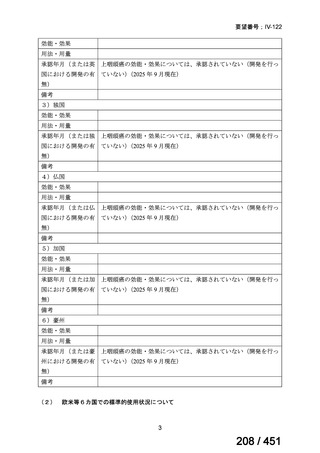
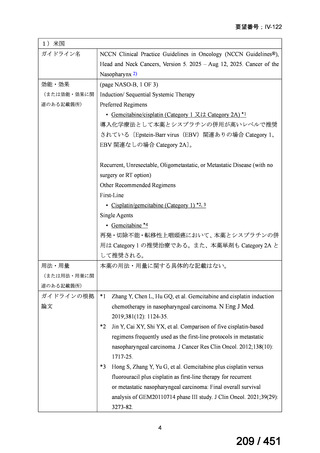
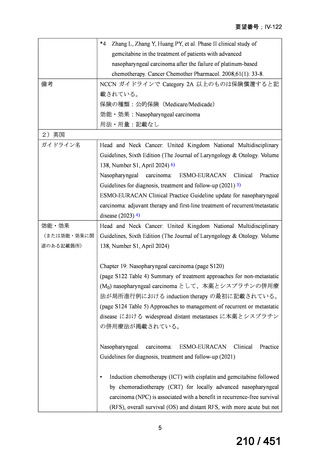
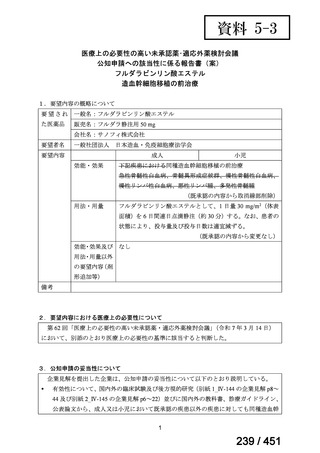
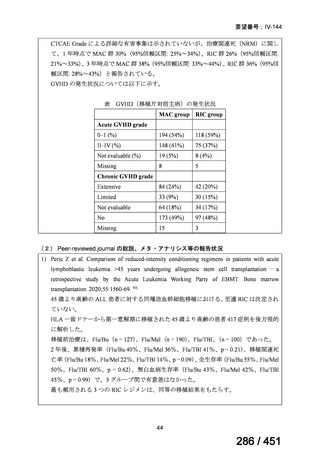
5. MDR-TB
5.1.2 Choice of medication
Recommendations
We strongly recommend, for the treatment of MDR-TB:
a. use of regimens that include bedaquiline, for all patients;
b. use of regimens that include linezolid, for all patients; and
c. use of regimens that include either levofloxacin or moxifloxacin, for all
patients (good evidence).
We strongly recommend, for the treatment of MDR-TB, against use of drugs
to which the infecting strain has drug susceptibility testing-proven resistance
(with the exception of high-dose isoniazid in the all-oral standardized shorter
regimen) (good evidence).
We conditionally recommend, for the treatment of MDR-TB, the following
five drugs as the initial regimen in the absence of drug susceptibility testingproven resistance or contraindications: (levofloxacin or moxifloxacin) AND
bedaquiline AND linezolid AND clofazimine AND cycloserine (poor
evidence).
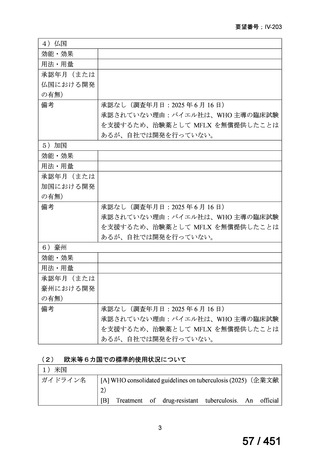
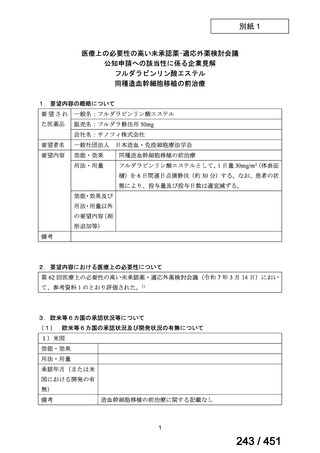
We conditionally recommend, for patients with less extensive MDR-TB
disease (smear negative, without cavitary lesions) that is solely pulmonary or
occurring at a site where TB is usually paucibacillary, that the initial regimen
could include only 4 drugs, consisting of (levofloxacin OR moxifloxacin)
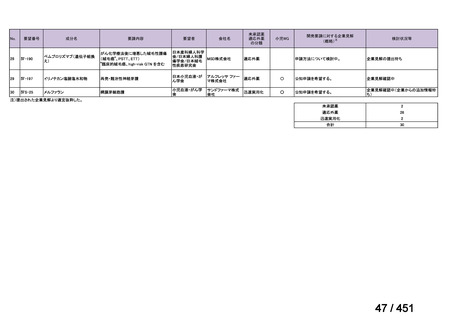
AND bedaquiline AND linezolid AND (clofazimine OR cycloserine) (poor
evidence).
We conditionally recommend, for the treatment of MDR-TB, that 5-to7 months after culture conversion occurs, any one of the drugs in the regimen
could be dropped, continuing the other 4; for patients whose initial phase
consisted of (levofloxacin OR moxifloxacin) AND bedaquiline AND linezolid
AND (clofazimine OR cycloserine), any one of the drugs can be dropped so
that the continuation phase consists of three drugs (poor evidence).
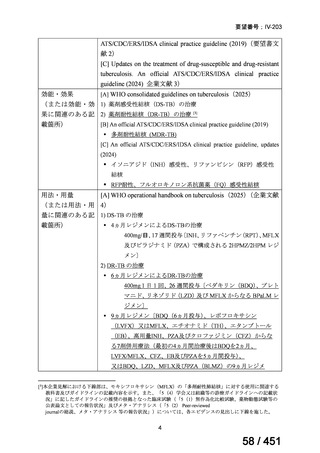
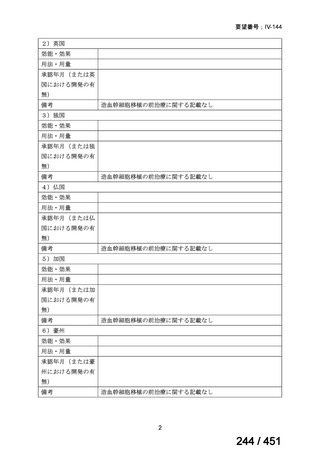
We conditionally recommend, for the treatment of MDR-TB, a total treatment
duration of 18 to 20 months, although this can be modified based on response
to therapy (poor evidence).
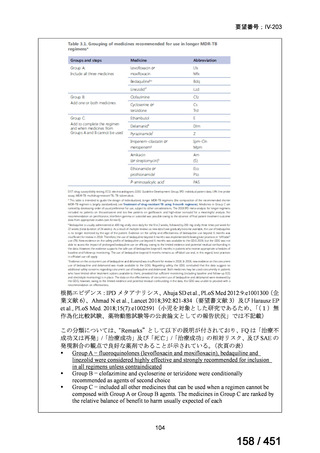
We conditionally recommend, for the treatment of pre-extensively drugresistant or extensively drug-resistant TB, or in situations where one or more
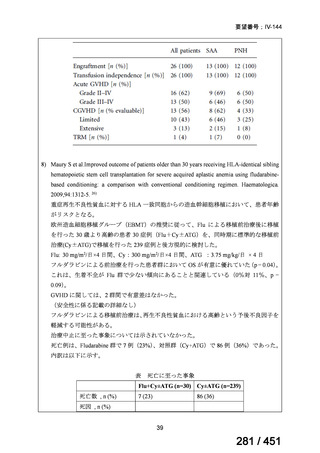
of the Group A and B drugs cannot be used due to side-effects, contraindications, unavailability or resistance, adding 1 or more Group C drugs to
ensure at least 5 drugs are in the regimen. The order of preference for the
addition of Group C drugs is (from most to least preferred): ethambutol,
114
168 / 451