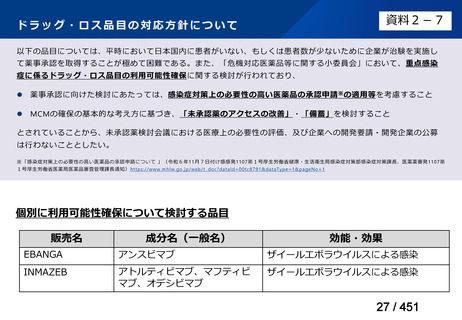
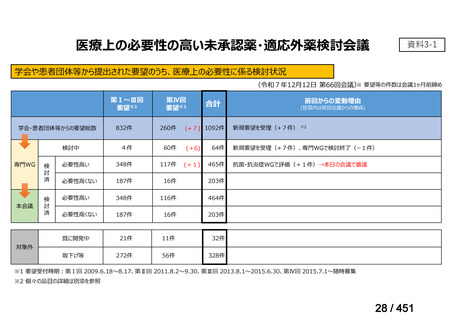
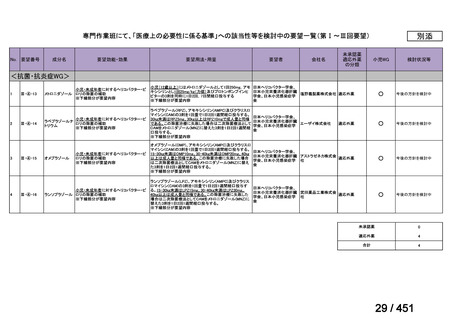
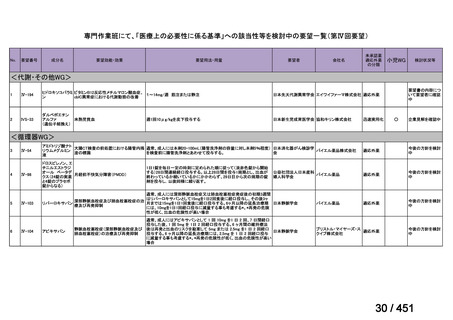
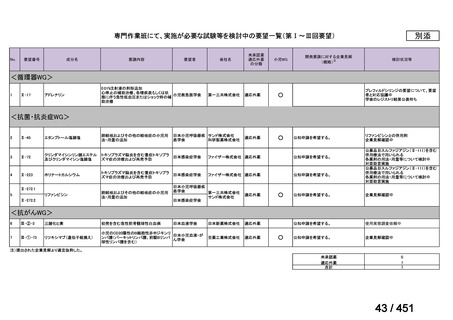
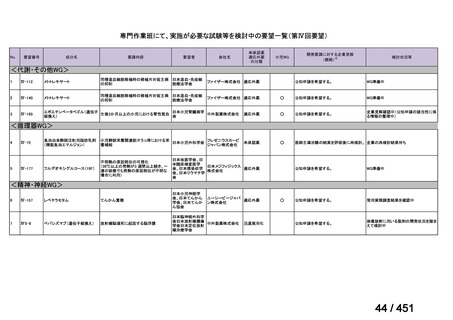
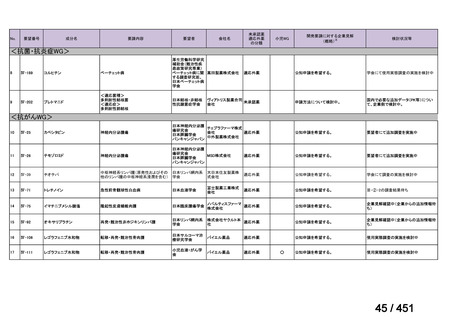
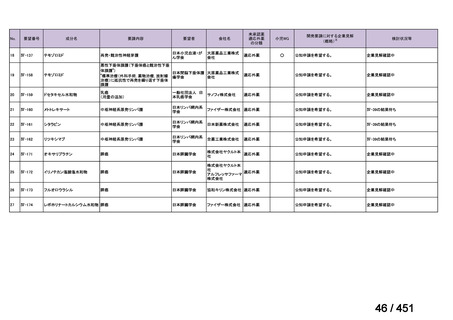
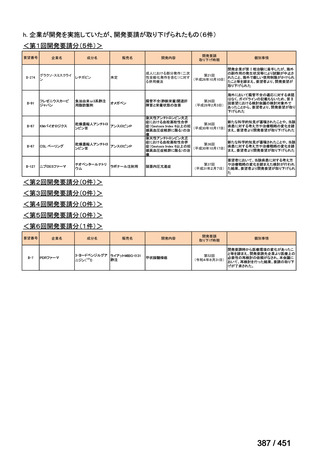
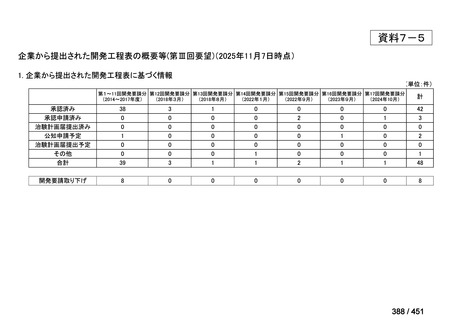
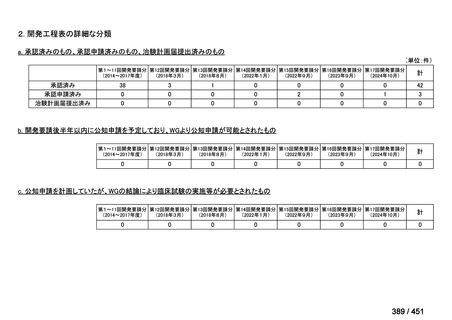
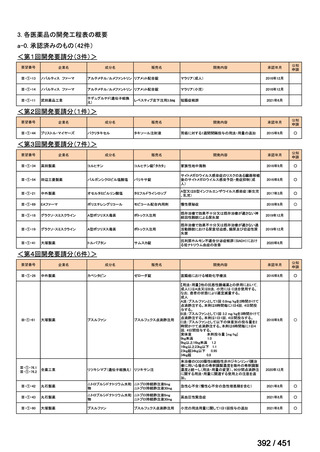
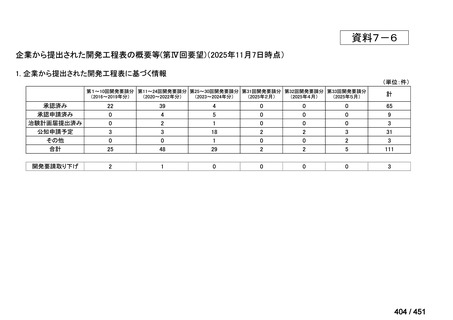
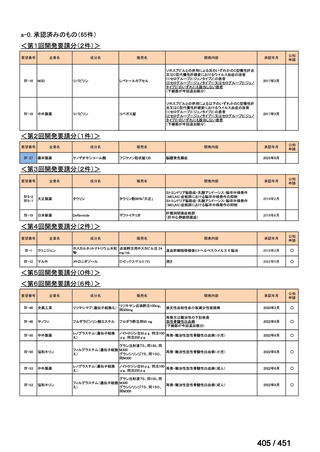
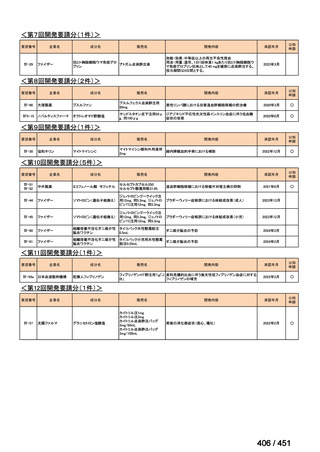
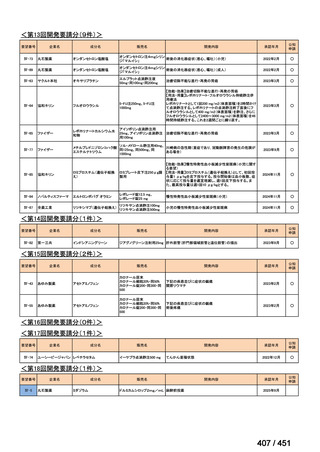
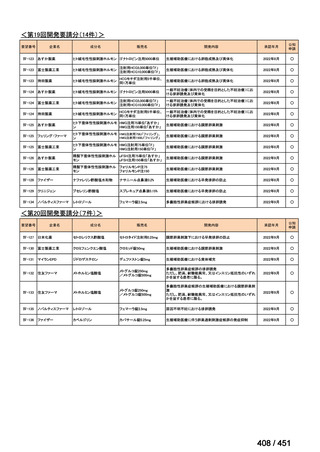
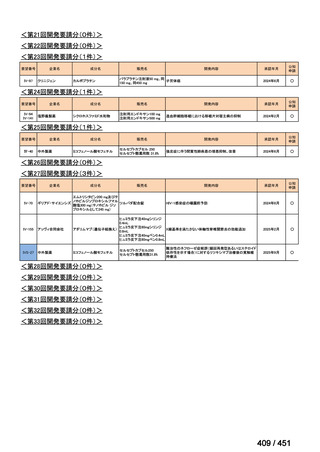
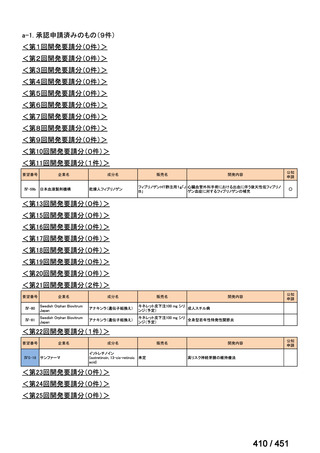
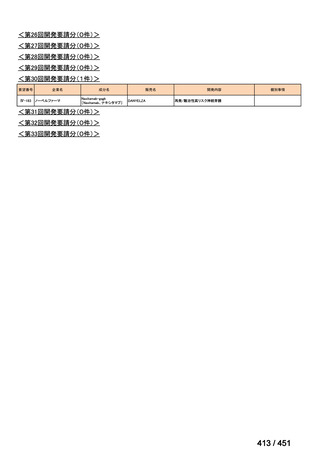
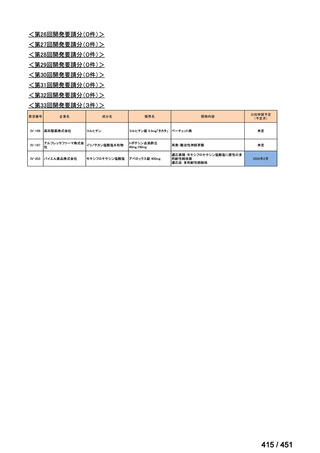
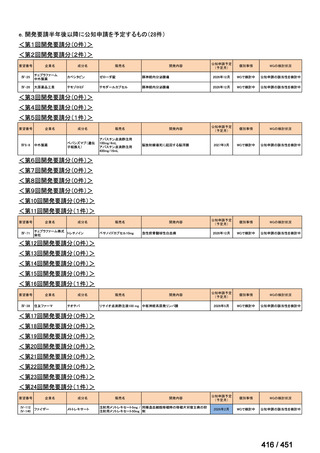
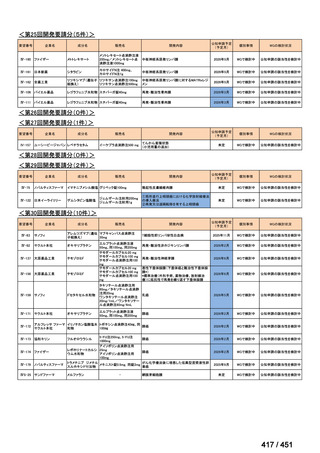
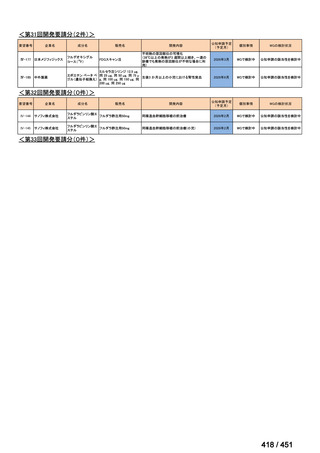
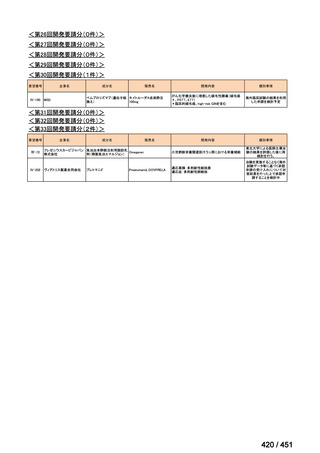
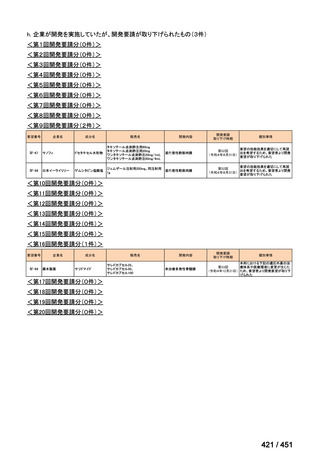
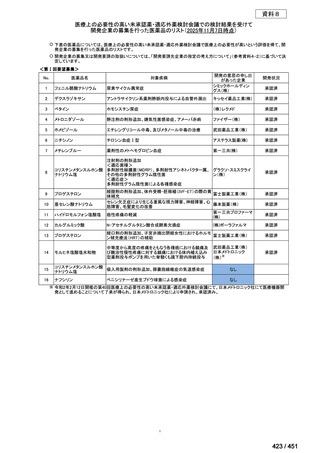
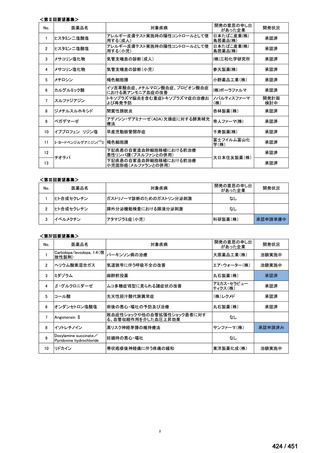
会議資料 (157 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00044.html |
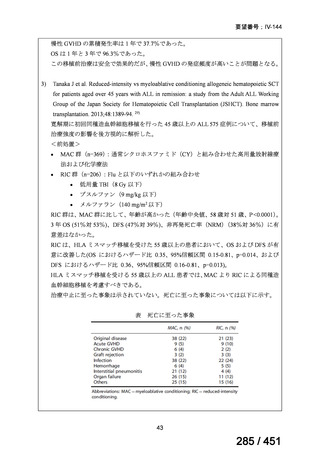
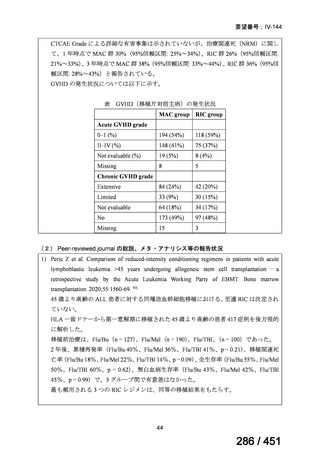
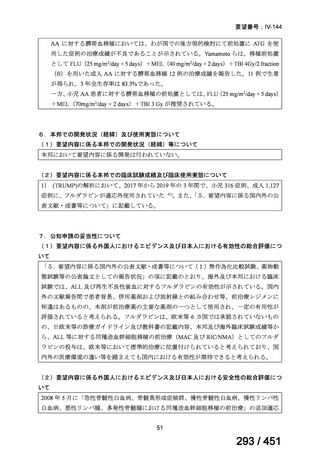
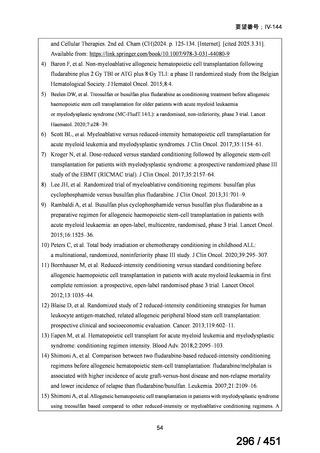
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第66回 12/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
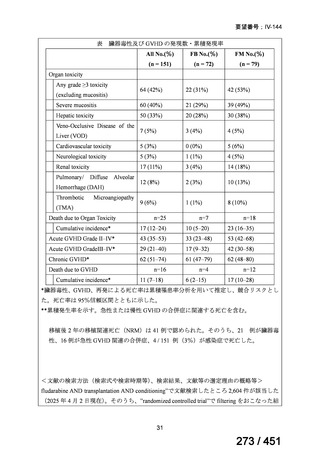
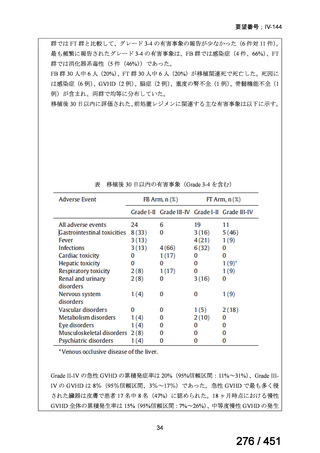
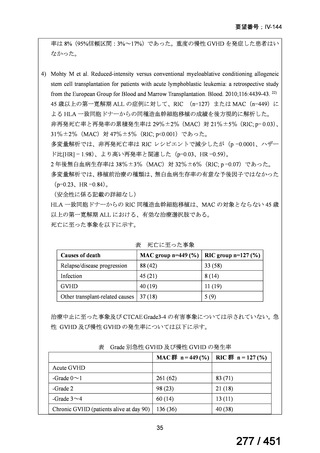
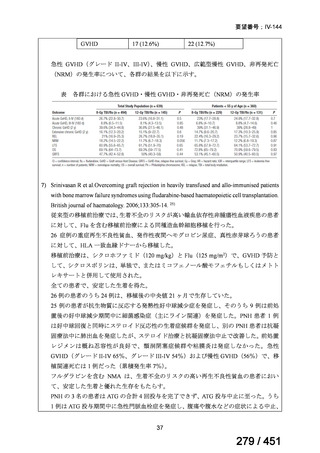
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
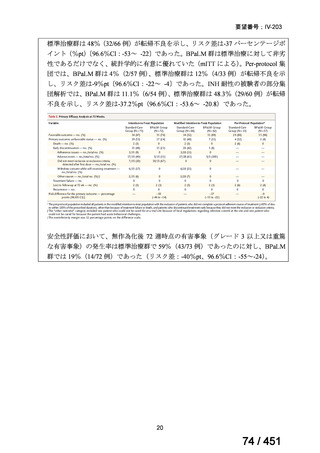
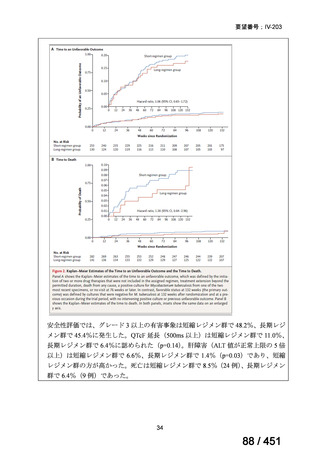
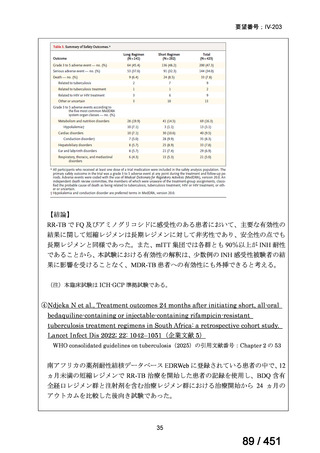
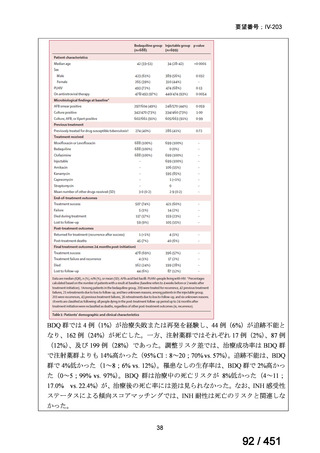
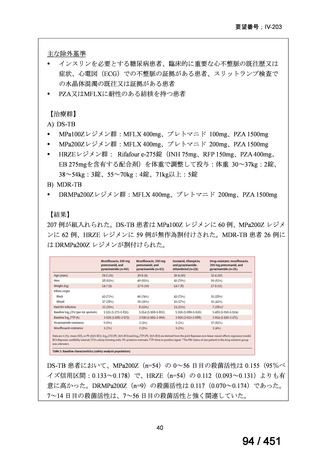
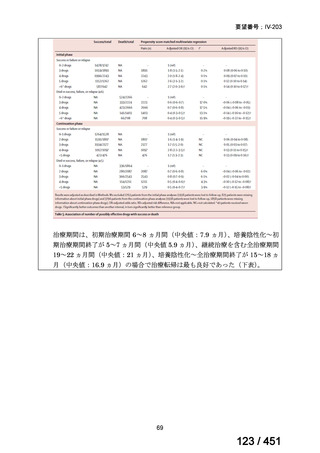
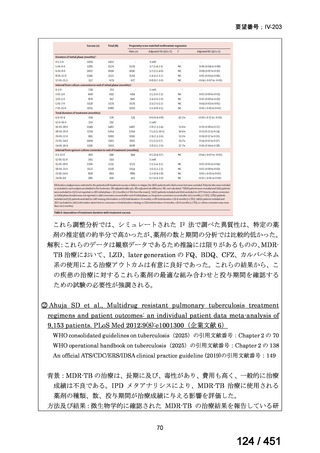
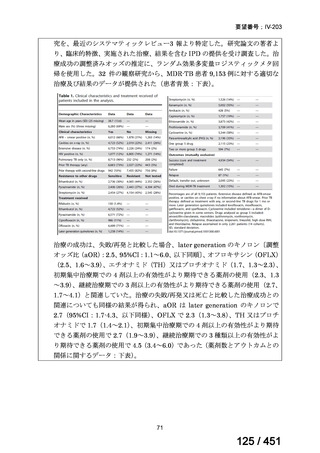
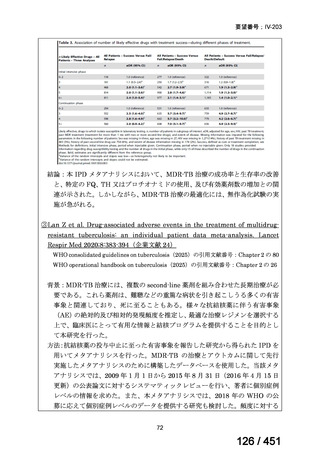
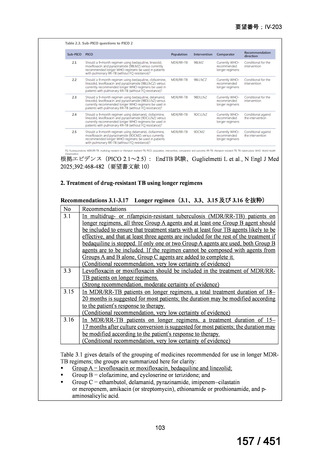
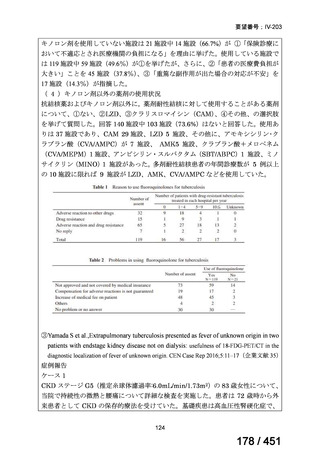
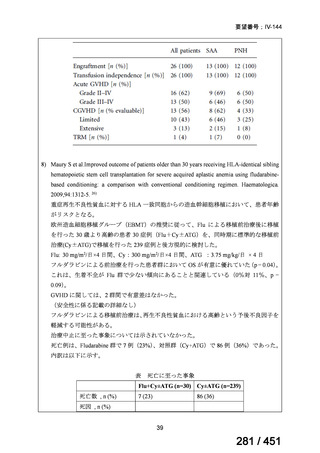
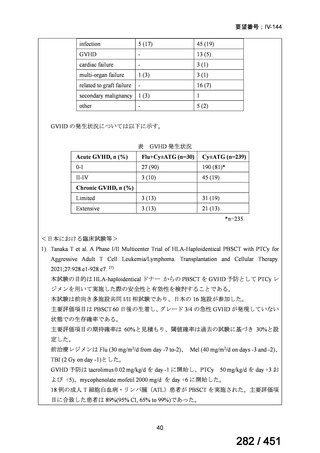
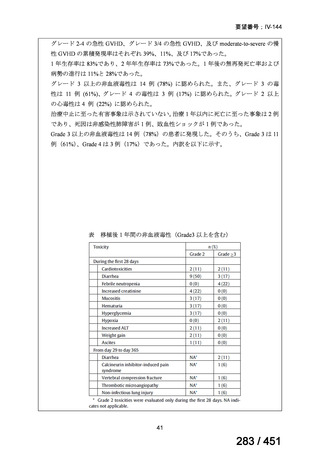
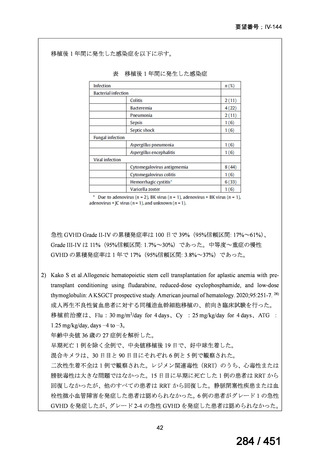
根拠エビデンス(PICO 2.1~2.5): EndTB 試験、Guglielmetti L et al., N Engl J Med
2025;392:468-482(要望書文献 10)
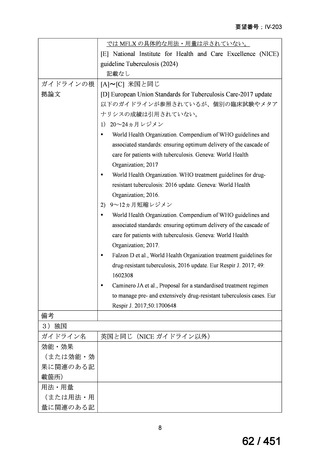
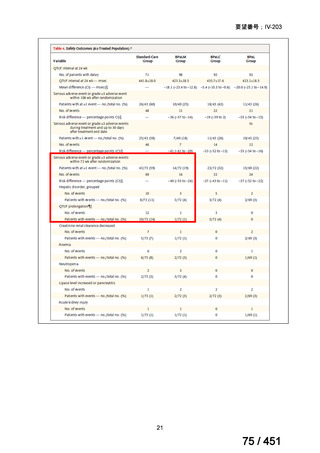
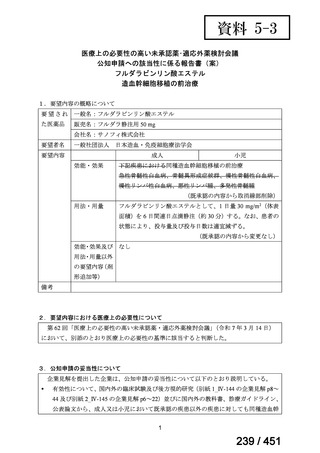
2. Treatment of drug-resistant TB using longer regimens
Recommendations 3.1-3.17 Longer regimen(3.1、3.3、3.15 及び 3.16 を抜粋)
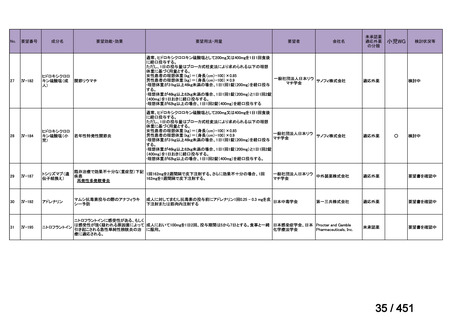
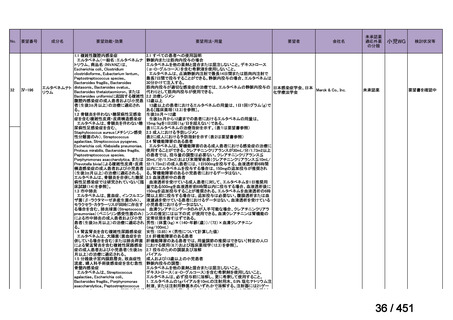
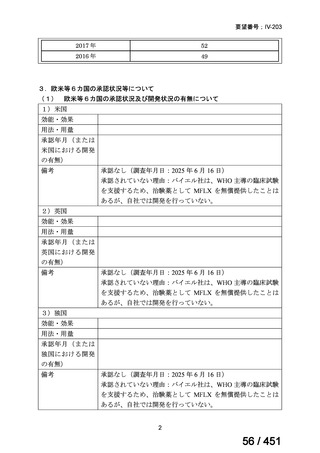
No
Recommendations
3.1
In multidrug- or rifampicin-resistant tuberculosis (MDR/RR-TB) patients on
longer regimens, all three Group A agents and at least one Group B agent should
be included to ensure that treatment starts with at least four TB agents likely to be
effective, and that at least three agents are included for the rest of the treatment if
bedaquiline is stopped. If only one or two Group A agents are used, both Group B
agents are to be included. If the regimen cannot be composed with agents from
Groups A and B alone, Group C agents are added to complete it.
(Conditional recommendation, very low certainty of evidence)
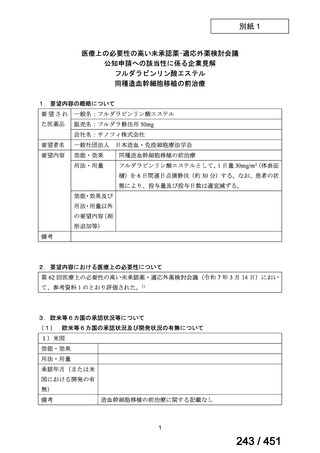
3.3
Levofloxacin or moxifloxacin should be included in the treatment of MDR/RRTB patients on longer regimens.
(Strong recommendation, moderate certainty of evidence)
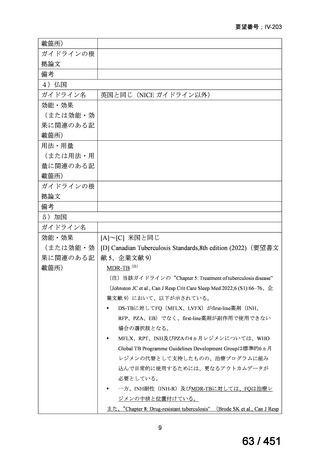
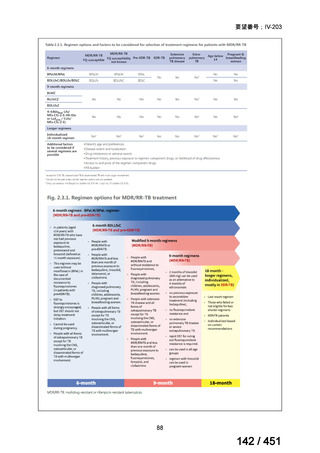
3.15 In MDR/RR-TB patients on longer regimens, a total treatment duration of 18–
20 months is suggested for most patients; the duration may be modified according
to the patient’s response to therapy.
(Conditional recommendation, very low certainty of evidence)
3.16 In MDR/RR-TB patients on longer regimens, a treatment duration of 15–
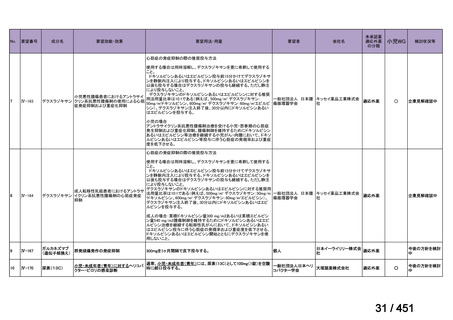
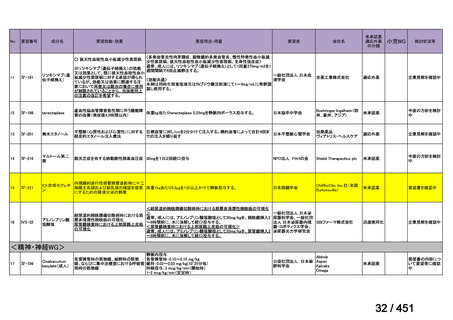
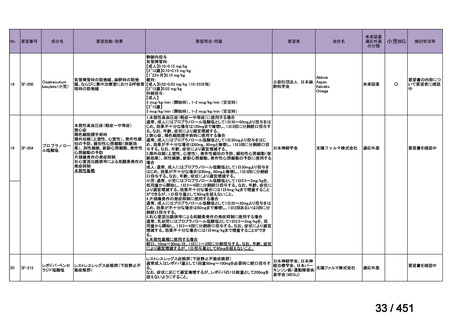
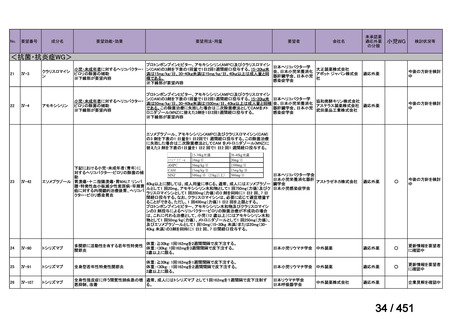
17 months after culture conversion is suggested for most patients; the duration may
be modified according to the patient’s response to therapy.
(Conditional recommendation, very low certainty of evidence)
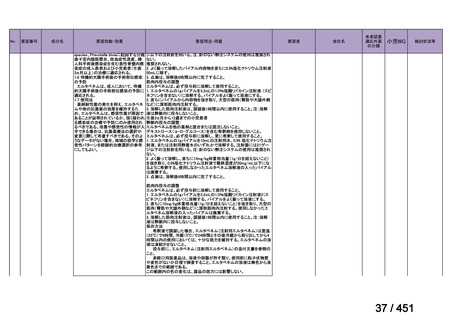
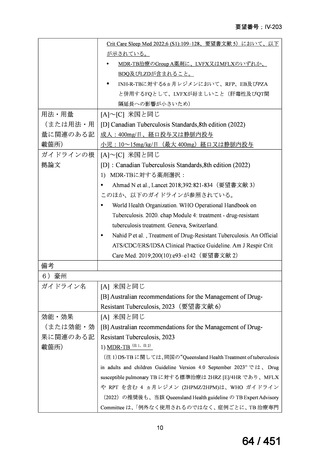
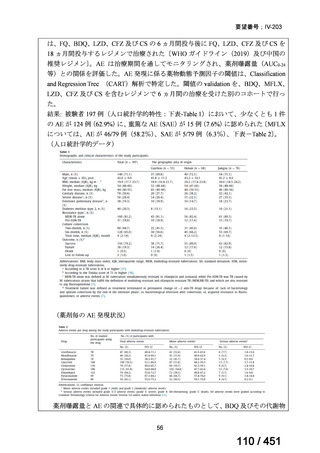
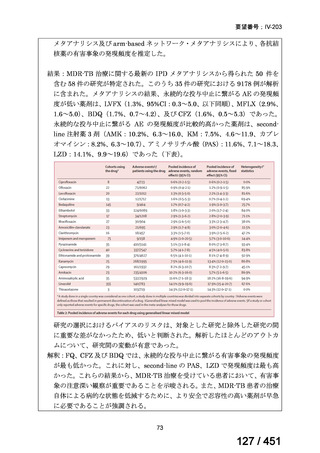
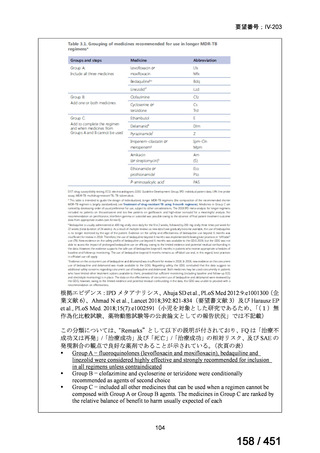
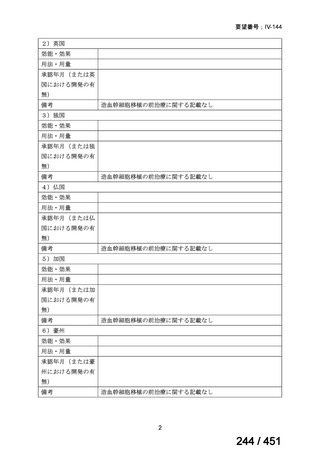
Table 3.1 gives details of the grouping of medicines recommended for use in longer MDRTB regimens; the groups are summarized here for clarity:
Group A = levofloxacin or moxifloxacin, bedaquiline and linezolid;
Group B = clofazimine, and cycloserine or terizidone; and
Group C = ethambutol, delamanid, pyrazinamide, imipenem–cilastatin
or meropenem, amikacin (or streptomycin), ethionamide or prothionamide, and paminosalicylic acid.
103
157 / 451