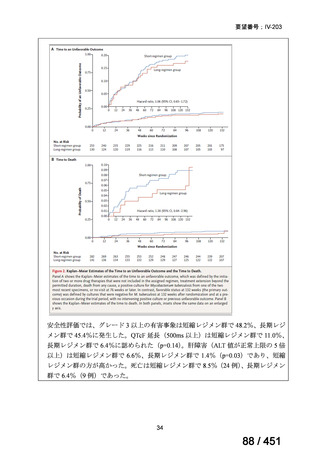
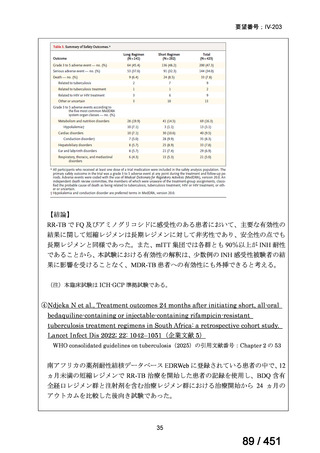
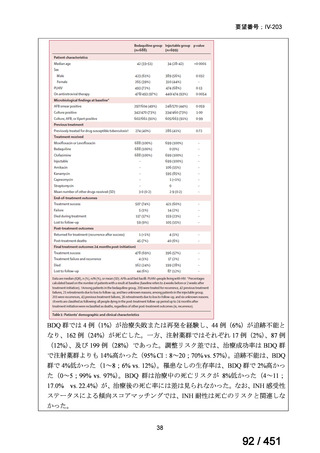
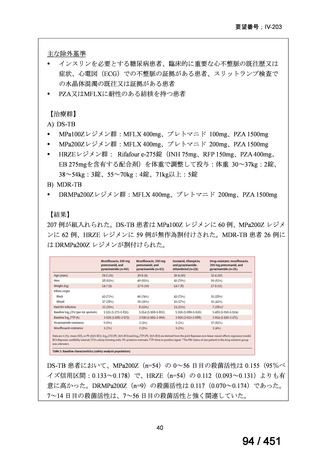
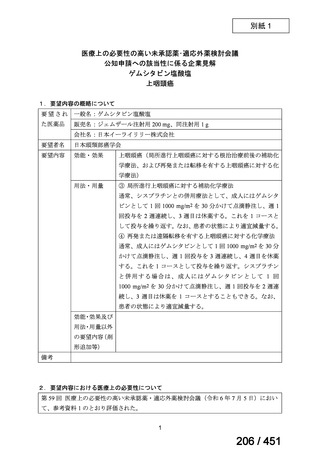
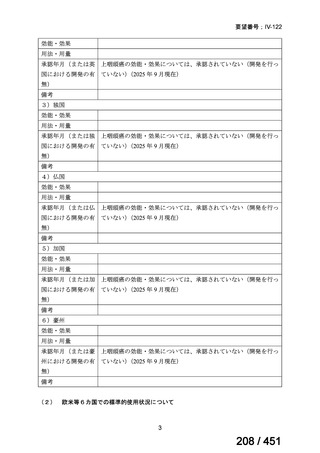
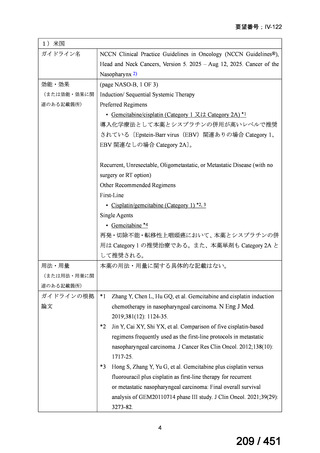
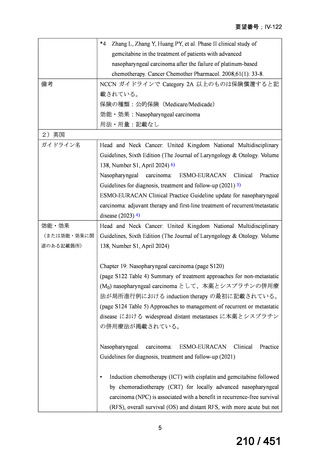
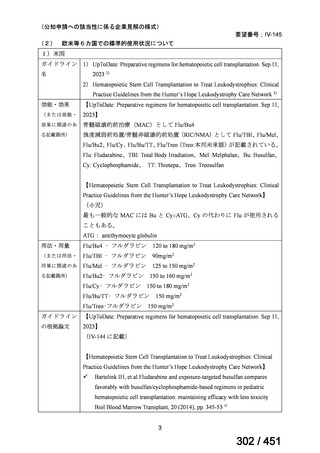
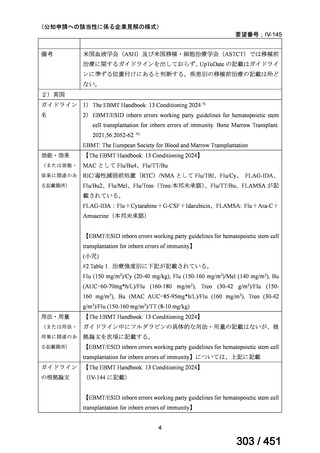
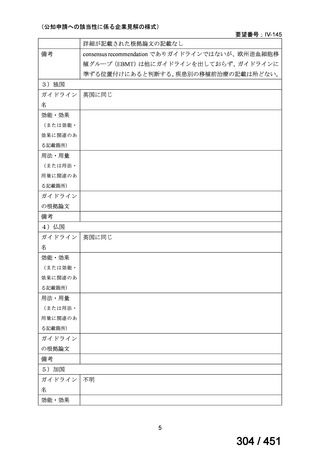
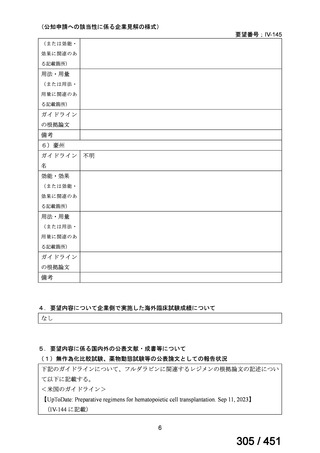
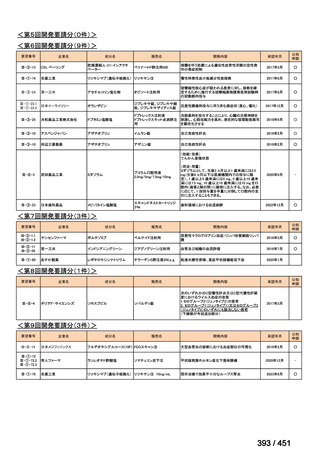
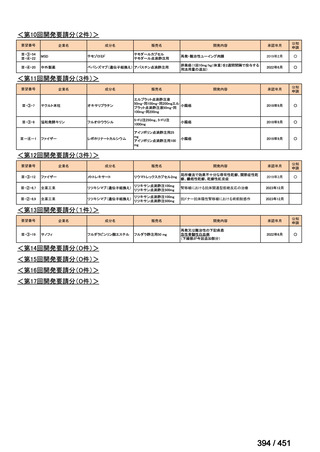
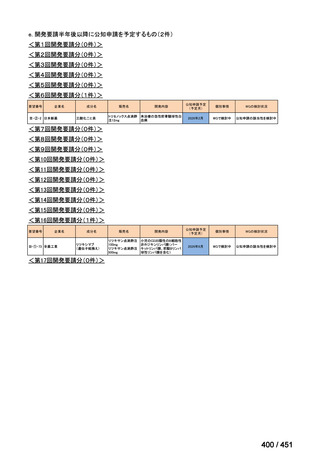
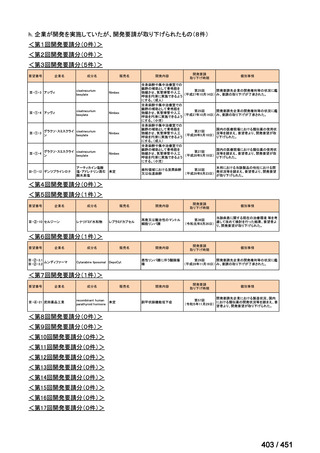
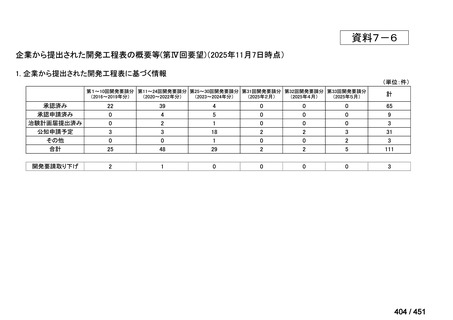
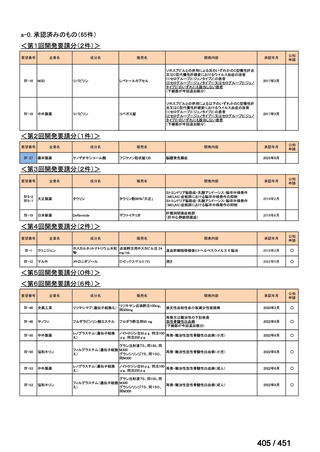
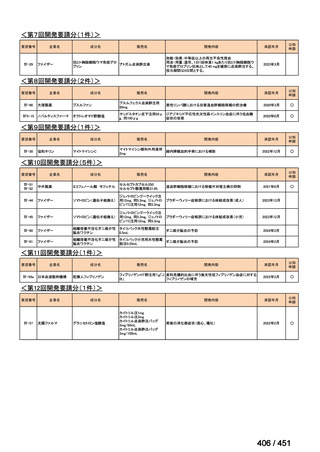
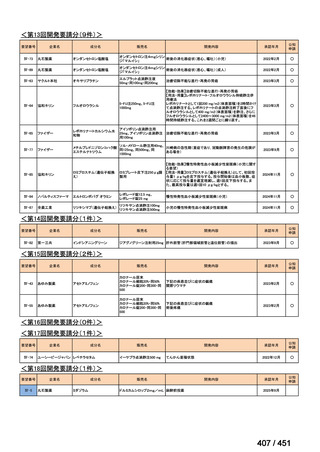
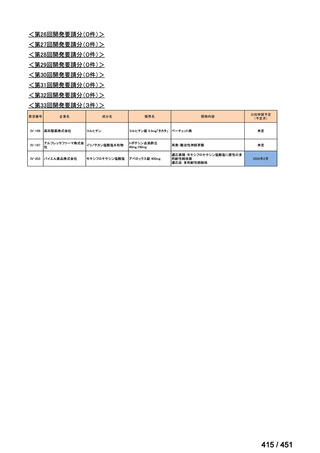
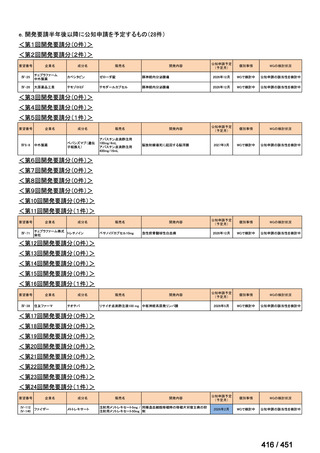
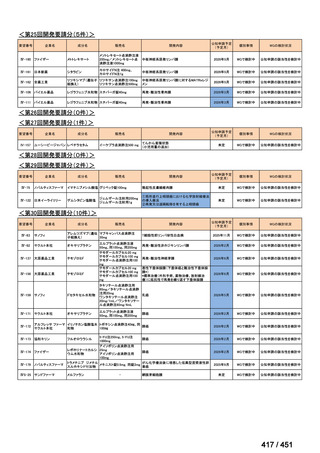
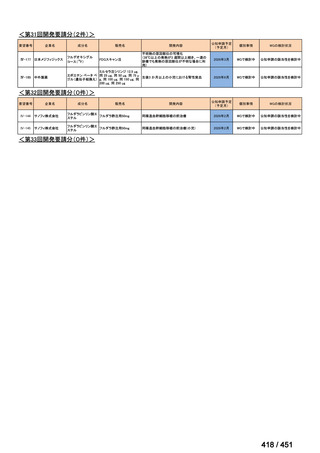
会議資料 (170 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00044.html |
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第66回 12/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
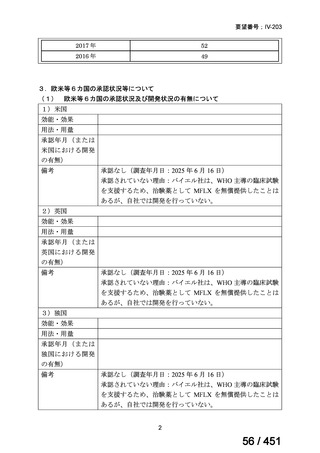
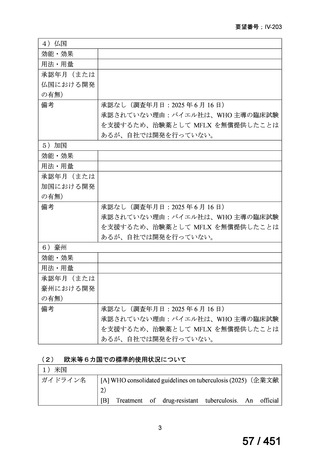
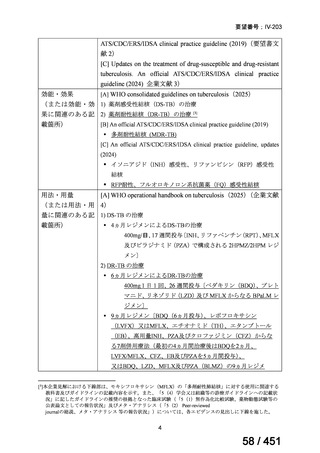
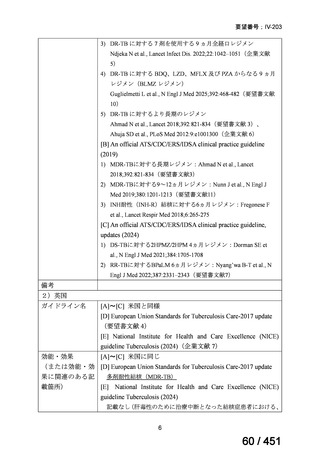
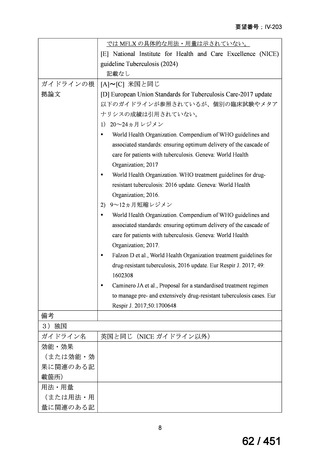
6)
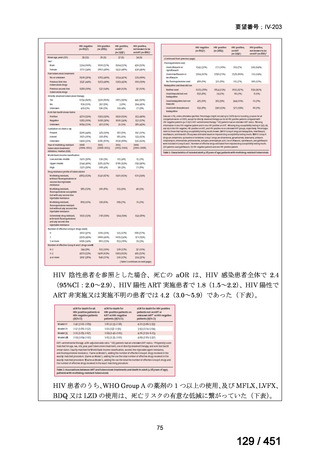
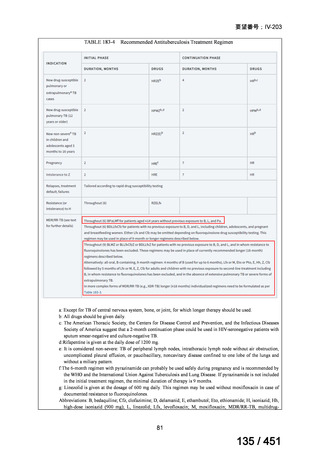
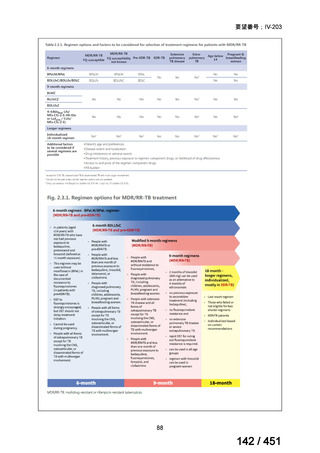
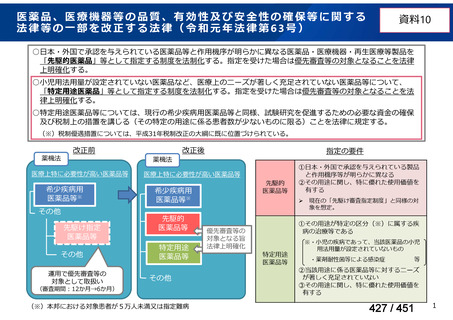
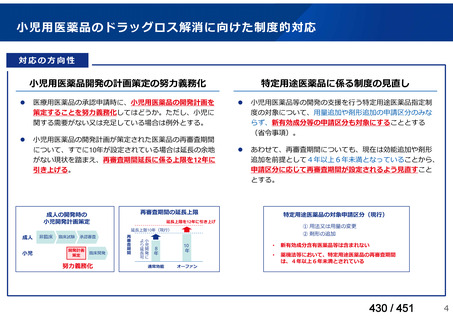
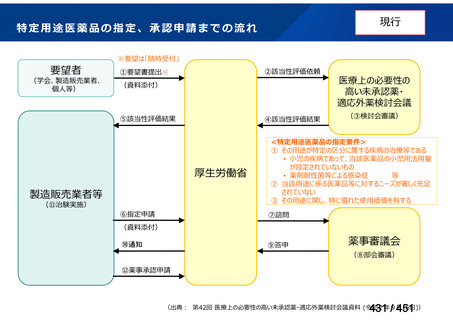
Australian recommendations for the Management of Drug-Resistant Tuberculosis,
2023(要望書文献6)
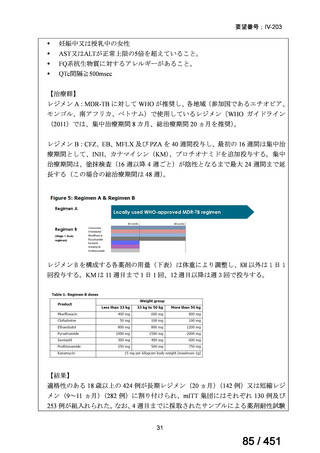
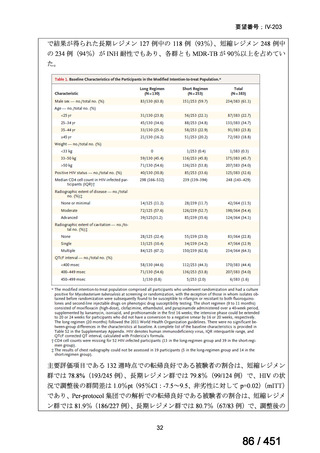
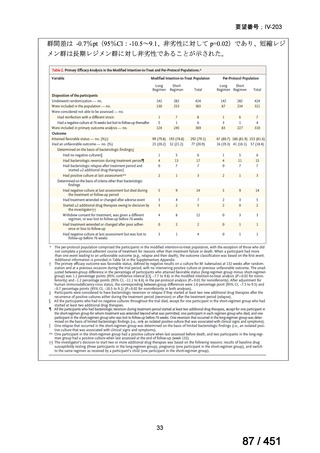
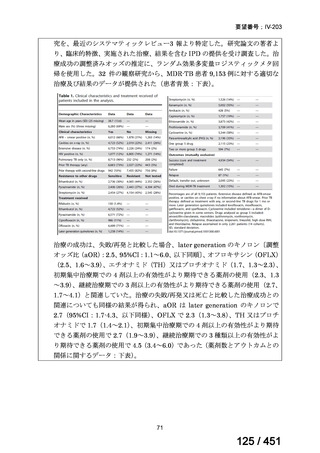
Multidrug-resistant TB treatment regimens
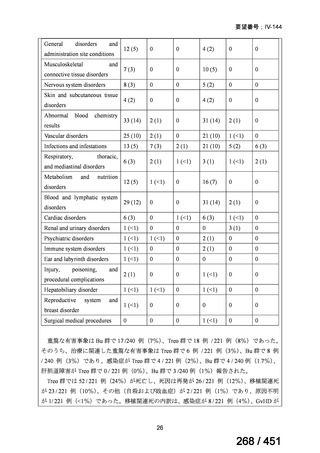
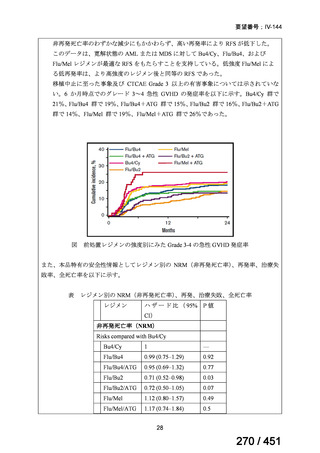
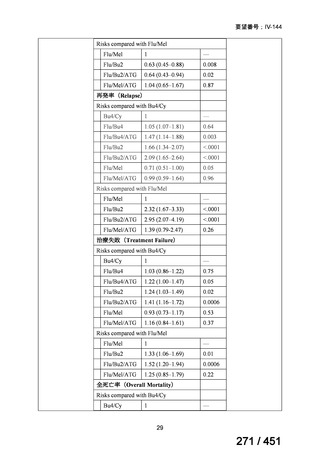
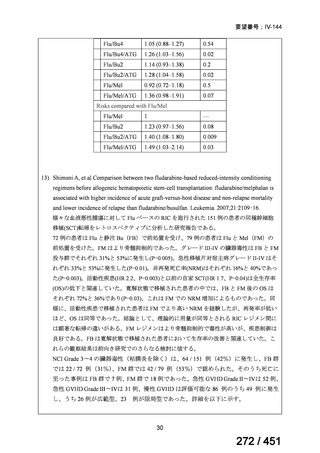
All patients diagnosed with multidrug-resistant tuberculosis (MDR-TB) can now be
considered for treatment with an all-oral shorter or longer course regimen. This also
applies to those with additional resistance to a fluoroquinolone. The WHO guidance
(2022) prioritises the use of a standardised shorter course regimen providing certain
criteria are met.
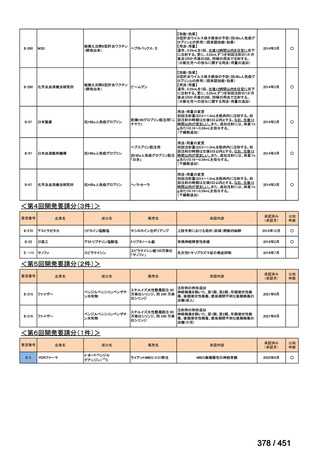
The most up to date options recommended by the WHO (2022) are:
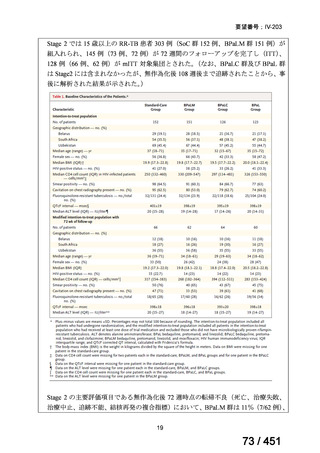
1. 6-month BPaLM regimen (fluoroquinolone susceptible):
comprises bedaquiline, pretomanid, linezolid (600 mg daily)
and moxifloxacin
is preferred to the 9–11 months shorter course or 18–20 months longer
course regimens
not suitable for those with previous exposure to bedaquiline, pretomanid,
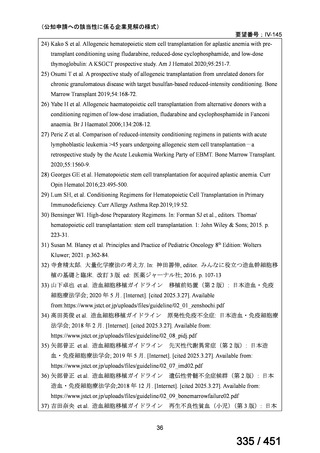
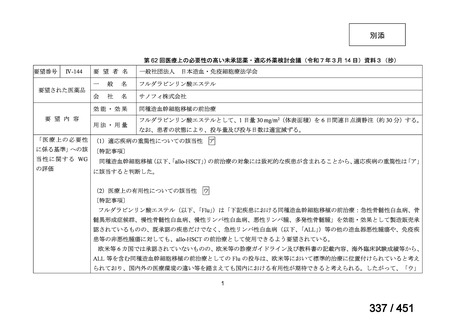
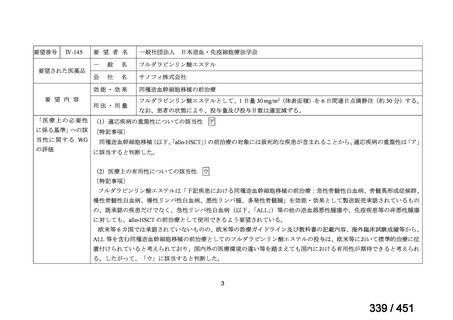
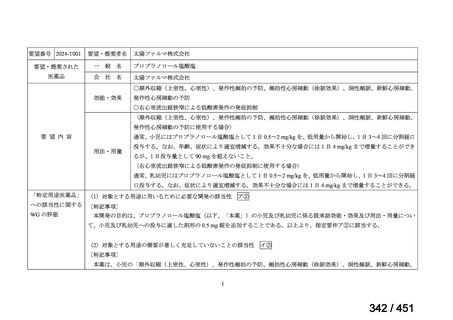
or linezolid for greater than one month unless resistance is excluded.
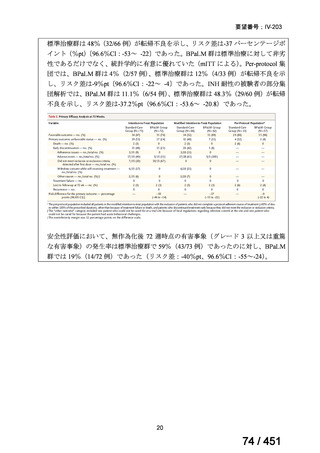
2. 6–9 month BPaL regimen (fluoroquinolone resistant):
comprises bedaquiline, pretomanid, linezolid (600 mg daily)
A 9-month regimen can be used if there is a slower, but still favorable,
treatment response
not suitable for those with previous exposure to bedaquiline, pretomanid,
or linezolid for greater than one month unless resistance is excluded.
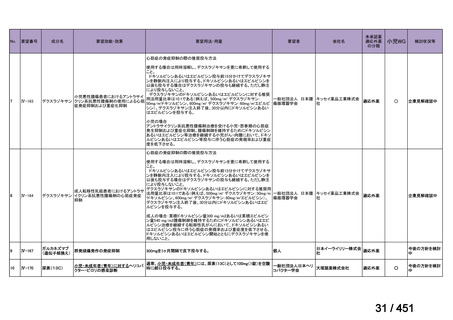
116
170 / 451