資料5-1 Ⅳ-203 モキシフロキサシン塩酸塩[15.1MB] (106 ページ)
出典
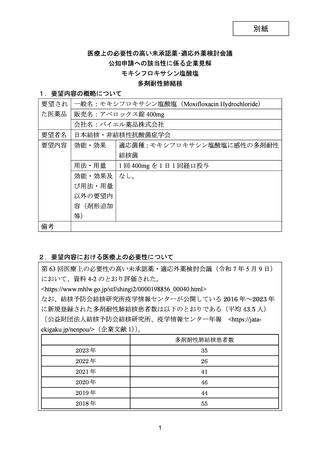
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00044.html |
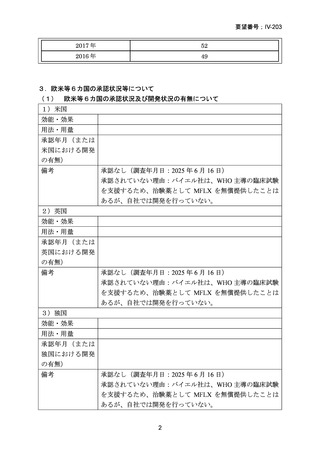
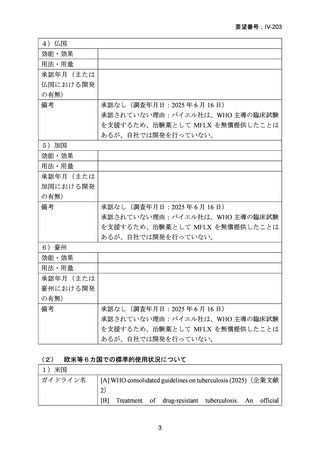
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第66回 12/12)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
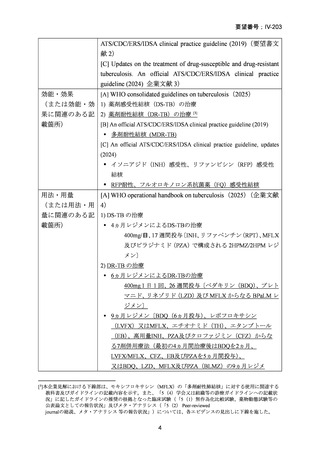
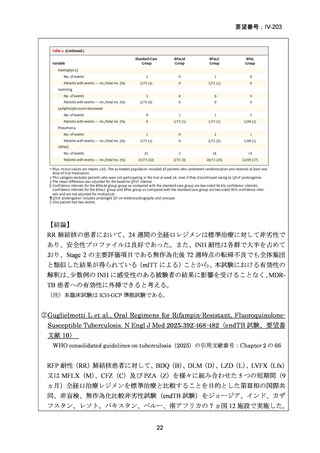
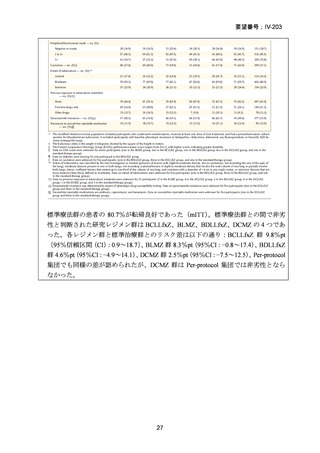
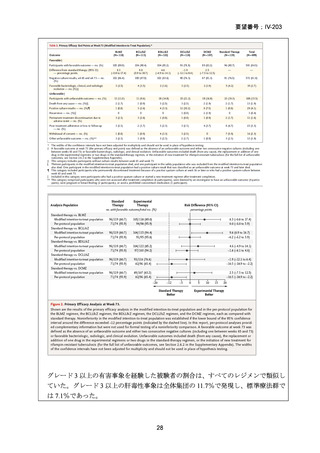
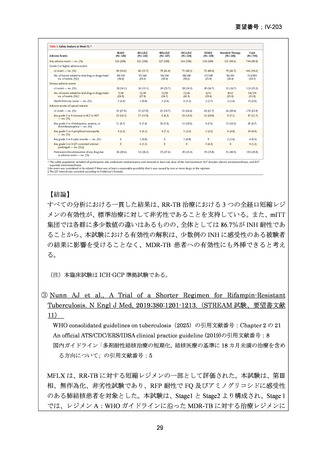
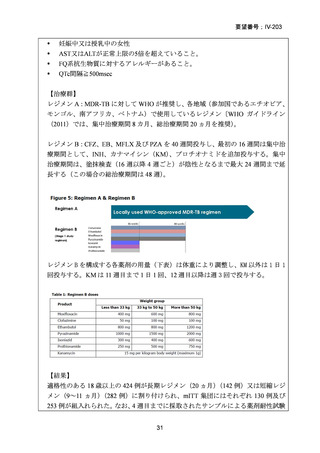
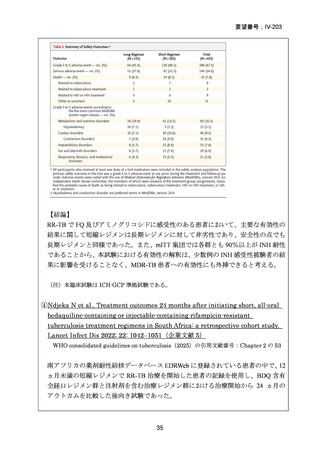
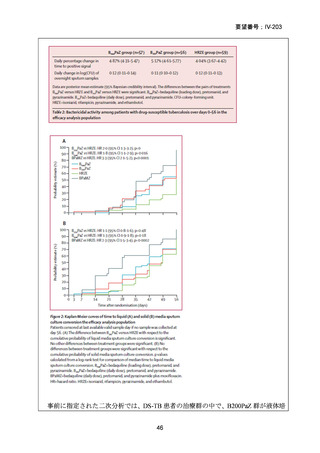
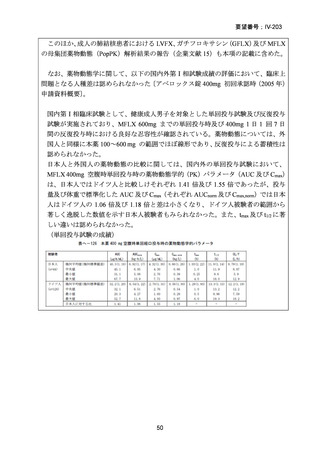
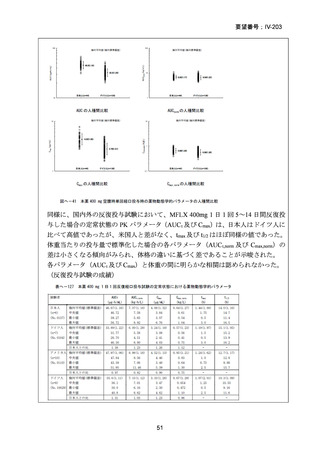
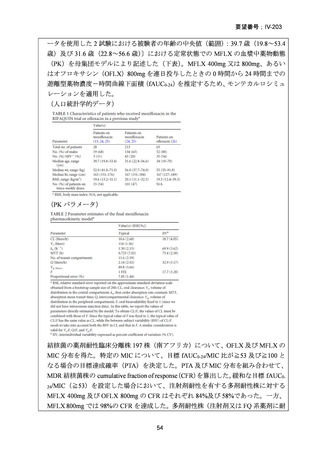
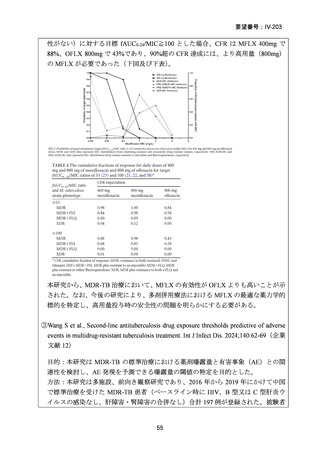
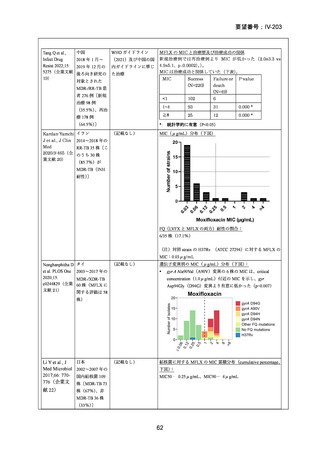
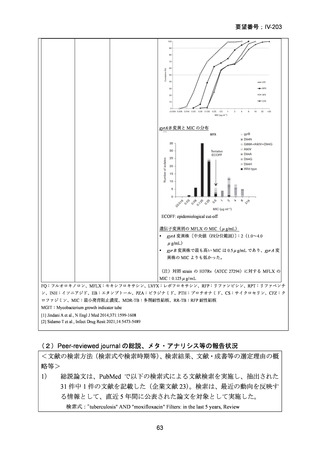
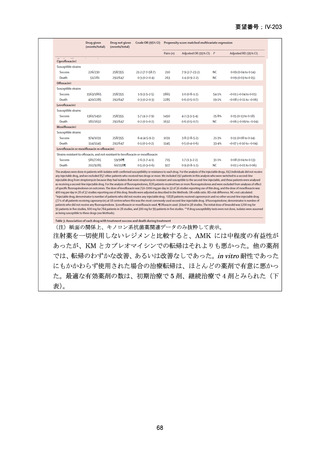
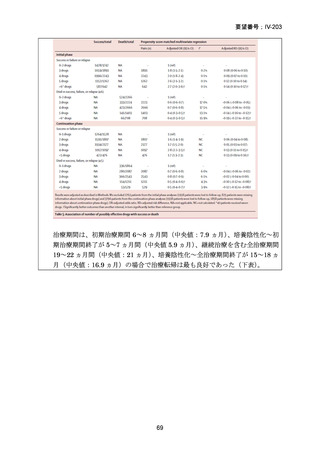
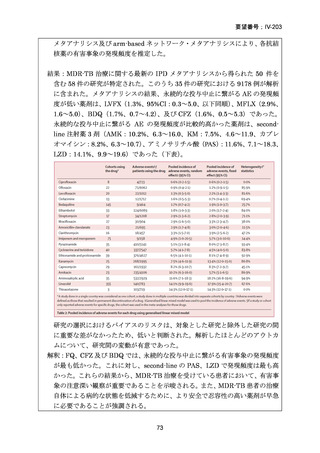
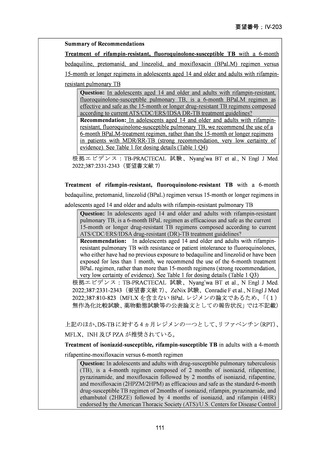
根 拠 エ ビ デ ン ス : 南 ア フ リ カ の 観 察 研 究 ( Ndjeka N et al., Lancet Infect Dis.
2022;22:1042–1051)(企業文献 5)
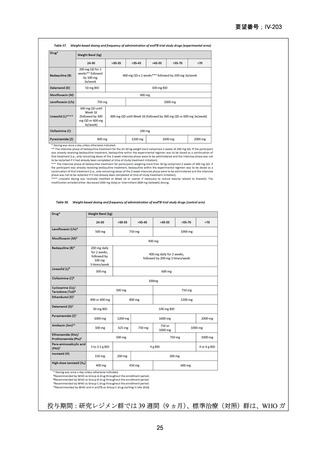
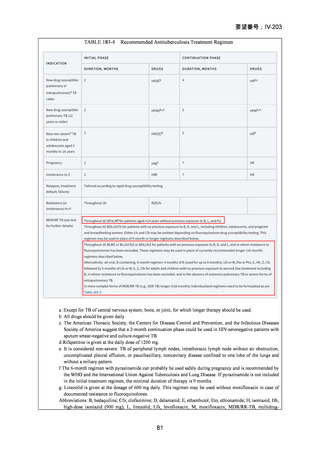
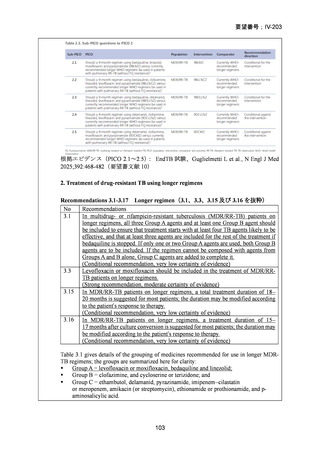
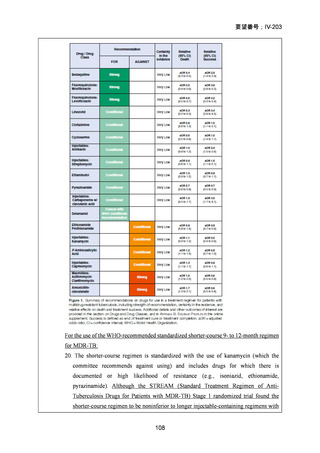
Recommendations 2.2 and 2.3 The modified 9-month all-oral regimens for MDR/RRTB
No
2.2
2.3
Recommendations (NEW)
WHO suggests using the 9-month all-oral regimens (BLMZ, BLLfxCZ and
BDLLfxZ) over currently recommended longer (>18 months) regimens in patients
with MDR/RR-TB and in whom resistance to fluoroquinolones has been excluded.
Among these regimens, using BLMZ is suggested over using BLLfxCZ, and
BLLfxCZ is suggested over BDLLfxZ.
(Conditional recommendation, very low certainty of evidence)
WHO suggests against using 9-month DCLLfxZ or DCMZ regimens compared
with currently recommended longer (>18 months) regimens in patients with
fluoroquinolone-susceptible MDR/RR-TB.
(Conditional recommendation, very low certainty of evidence)
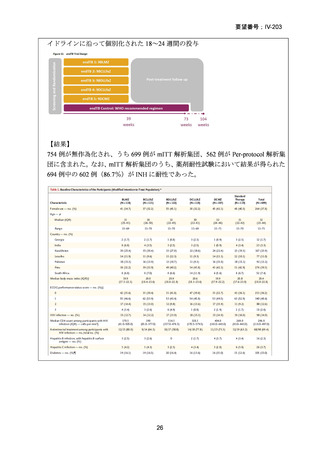
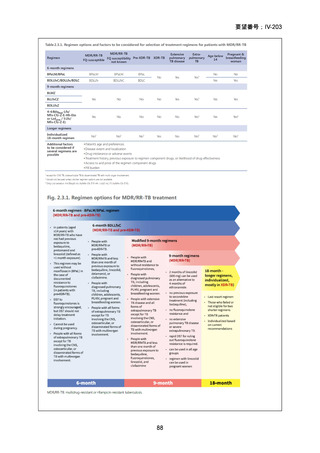
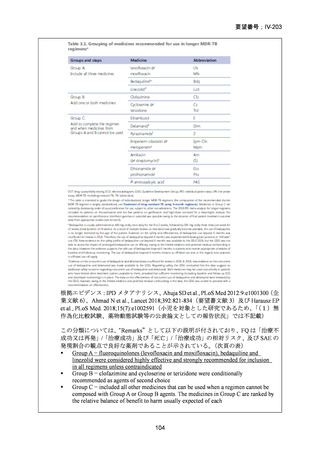
Remarks
1. The recommended modified 9-month all-oral regimens comprise bedaquiline, linezolid and
pyrazinamide in different combinations with levofloxacin/moxifloxacin, clofazimine and
delamanid.
2. This recommendation applies to the following:
a) People with MDR/RR-TB and in whom resistance to fluoroquinolones has been
excluded.
b) People with diagnosed pulmonary TB, including children, adolescents, people libing
with HIV (PLHIV), and pregnant and breastfeeding women.
c) People with extensive TB disease and all forms of extrapulmonary TB except for TB
involving the CNS, osteoarticular TB or disseminated forms of TB with multiorgan
involvement.
d) People with MDR/RR-TB and less than 1 month of previous exposure to any of the
component medicines of the regimen (apart from pyrazinamide and fluoroquinolones).
When exposure is greater than 1 month, these patients may still receive one of the
regimens if resistance to the specific medicines with such exposure has been ruled out.
e) Children and adolescents who do not have bacteriological confirmation of TB or
resistance patterns but do have a high likelihood of MDR/RR-TB (based on clinical
signs and symptoms of TB, in combination with a history of contact with a patient with
confirmed MDR/RR-TB).
102