よむ、つかう、まなぶ。
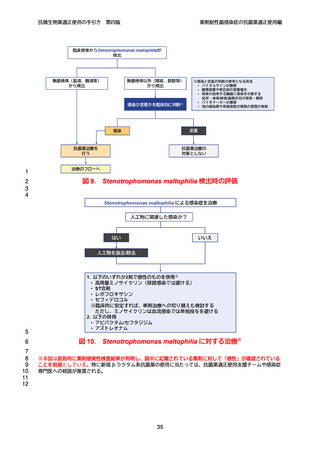
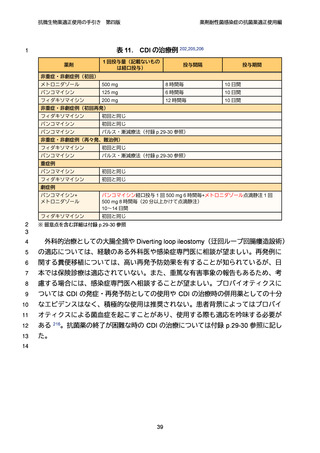
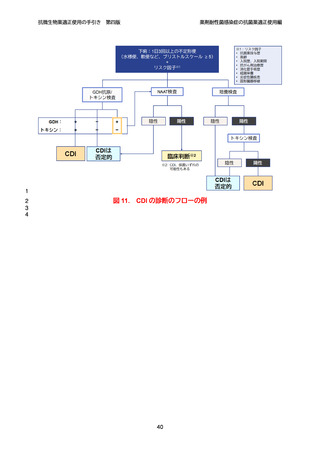
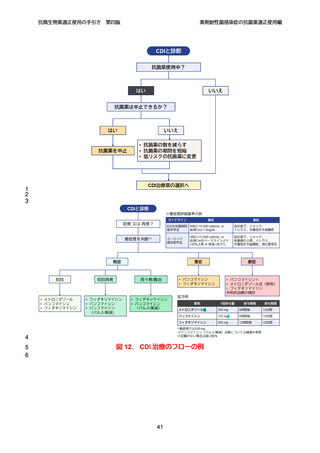
【参考資料2-3】抗微生物薬適正使用の手引き 第四版(案)薬剤耐性菌感染症の抗菌薬適正使用編 (97 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64503.html |
| 出典情報 | 厚生科学審議会 感染症部会(第99回 10/21)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
抗微生物薬適正使用の手引き
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
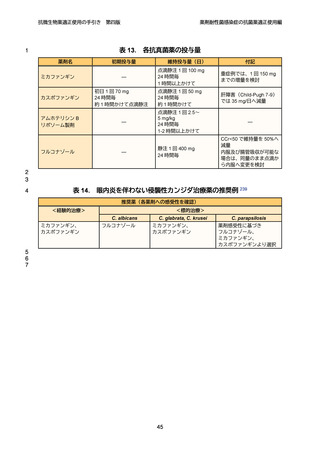
1
2
3
135. Papp-Wallace KM, et al. a Broad-Spectrum Serine β-lactamase Inhibitor, Restores
Sulbactam Activity Against Acinetobacter Species. Clin Infect Dis. 2023. 76(Suppl
2):S194-S201.
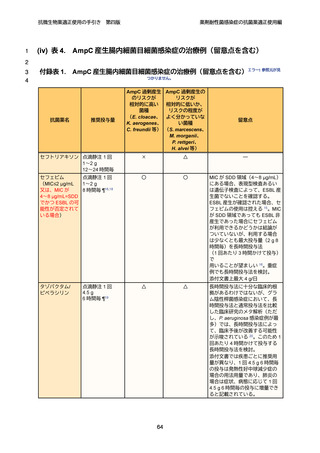
4
5
6
7
136. Kaye KS, et al. Efficacy and safety of sulbactam-durlobactam versus colistin for the
treatment of patients with serious infections caused by Acinetobacter baumanniicalcoaceticus complex: a multicentre, randomised, active-controlled, phase 3, noninferiority clinical trial (ATTACK). Lancet Infect Dis. 2023. 23(9):1072-1084.
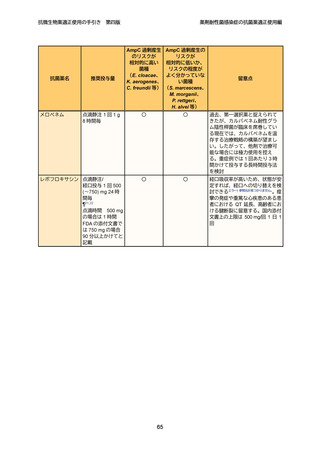
8
9
10
137. Miller AA, et al. Characterization of Acinetobacter baumannii-calcoaceticus complex
isolates and microbiological outcome for patients treated with sulbactam-durlobactam in
a phase 3 trial (ATTACK). Antimicrob Agents Chemother. 2024. 68(5):e0169823.
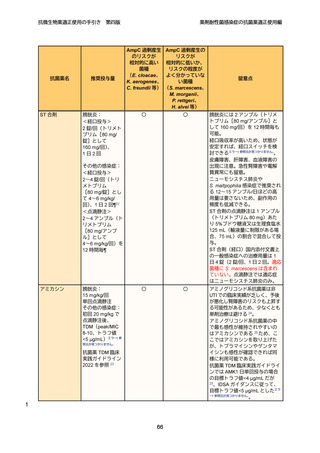
11
12
13
138. Alosaimy S, et al. Clinical Outcomes of Eravacycline in Patients Treated Predominately
for Carbapenem-Resistant Acinetobacter baumannii. Microbiol Spectr. 2022.
10(5):e0047922.
14
15
139. Isler B, et al. New Treatment Options against Carbapenem-Resistant Acinetobacter
baumannii Infections. Antimicrob Agents Chemother. 2018 Dec;63(1):e01110-18.
16
17
140. Doi Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections.
Clin Infect Dis. 2019. 69(Suppl 7):S565-S575.
18
19
20
141. Khan A, et al. Evaluation of the Performance of Manual Antimicrobial Susceptibility
Testing Methods and Disk Breakpoints for Stenotrophomonas maltophilia. Antimicrob
Agents Chemother. 2023. 95(5):e02631-20.
21
22
23
142. Khan A, et al. Evaluation of the Vitek 2, Phoenix, and MicroScan for Antimicrobial
Susceptibility Testing of Stenotrophomonas maltophilia. J Clin Microbiol. 2021.
59(9):e0065421.
24
25
143. Mojica MF, et al. Clinical challenges treating Stenotrophomonas maltophilia infections:
an update. JAC Antimicrob Resist. 2022. 4(3):dlac040.
26
27
144. Brooke JS. Advances in the Microbiology of Stenotrophomonas maltophilia. Clin
Microbiol Rev. 2021. 34(3):e0003019.
28
29
30
145. Garcia-Leon G, et al. High-level quinolone resistance is associated with the
overexpression of smeVWX in Stenotrophomonas maltophilia clinical isolates. Clin
Microbiol Infect. 2015. 21(5):464-467.
31
32
146. Garcia-Leon G, et al. Interplay between intrinsic and acquired resistance to quinolones
in Stenotrophomonas maltophilia. Environ Microbiol. 2014. 16(5):1282-1296.
33
34
35
147. Toleman MA, et al. Global emergence of trimethoprim/sulfamethoxazole resistance in
Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg Infect Dis.
2007. 13(4):559-65.
36
37
38
148. Hu LF, et al. Stenotrophomonas maltophilia resistance to trimethoprim/sulfamethoxazole
mediated by acquisition of sul and dfrA genes in a plasmid-mediated class 1 integron. Int
J Antimicrob Agents. 2011. 37(3):230-234.
39
40
149. Hase R, et al. Clinical characteristics and genome epidemiology of Stenotrophomonas
maltophilia in Japan. J Antimicrob Chemother. 2024. 79(8):1843-1855.
41
42
43
150. Sakoh T, et al. Cefiderocol susceptibility of 146 Stenotrophomonas maltophilia strains
clinically isolated from blood in two Japanese hospitals over a 10-year period. Eur J Clin
Microbiol Infect Dis. 2024. 43(12):2485-2488.
97
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
1
2
3
135. Papp-Wallace KM, et al. a Broad-Spectrum Serine β-lactamase Inhibitor, Restores
Sulbactam Activity Against Acinetobacter Species. Clin Infect Dis. 2023. 76(Suppl
2):S194-S201.
4
5
6
7
136. Kaye KS, et al. Efficacy and safety of sulbactam-durlobactam versus colistin for the
treatment of patients with serious infections caused by Acinetobacter baumanniicalcoaceticus complex: a multicentre, randomised, active-controlled, phase 3, noninferiority clinical trial (ATTACK). Lancet Infect Dis. 2023. 23(9):1072-1084.
8
9
10
137. Miller AA, et al. Characterization of Acinetobacter baumannii-calcoaceticus complex
isolates and microbiological outcome for patients treated with sulbactam-durlobactam in
a phase 3 trial (ATTACK). Antimicrob Agents Chemother. 2024. 68(5):e0169823.
11
12
13
138. Alosaimy S, et al. Clinical Outcomes of Eravacycline in Patients Treated Predominately
for Carbapenem-Resistant Acinetobacter baumannii. Microbiol Spectr. 2022.
10(5):e0047922.
14
15
139. Isler B, et al. New Treatment Options against Carbapenem-Resistant Acinetobacter
baumannii Infections. Antimicrob Agents Chemother. 2018 Dec;63(1):e01110-18.
16
17
140. Doi Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections.
Clin Infect Dis. 2019. 69(Suppl 7):S565-S575.
18
19
20
141. Khan A, et al. Evaluation of the Performance of Manual Antimicrobial Susceptibility
Testing Methods and Disk Breakpoints for Stenotrophomonas maltophilia. Antimicrob
Agents Chemother. 2023. 95(5):e02631-20.
21
22
23
142. Khan A, et al. Evaluation of the Vitek 2, Phoenix, and MicroScan for Antimicrobial
Susceptibility Testing of Stenotrophomonas maltophilia. J Clin Microbiol. 2021.
59(9):e0065421.
24
25
143. Mojica MF, et al. Clinical challenges treating Stenotrophomonas maltophilia infections:
an update. JAC Antimicrob Resist. 2022. 4(3):dlac040.
26
27
144. Brooke JS. Advances in the Microbiology of Stenotrophomonas maltophilia. Clin
Microbiol Rev. 2021. 34(3):e0003019.
28
29
30
145. Garcia-Leon G, et al. High-level quinolone resistance is associated with the
overexpression of smeVWX in Stenotrophomonas maltophilia clinical isolates. Clin
Microbiol Infect. 2015. 21(5):464-467.
31
32
146. Garcia-Leon G, et al. Interplay between intrinsic and acquired resistance to quinolones
in Stenotrophomonas maltophilia. Environ Microbiol. 2014. 16(5):1282-1296.
33
34
35
147. Toleman MA, et al. Global emergence of trimethoprim/sulfamethoxazole resistance in
Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg Infect Dis.
2007. 13(4):559-65.
36
37
38
148. Hu LF, et al. Stenotrophomonas maltophilia resistance to trimethoprim/sulfamethoxazole
mediated by acquisition of sul and dfrA genes in a plasmid-mediated class 1 integron. Int
J Antimicrob Agents. 2011. 37(3):230-234.
39
40
149. Hase R, et al. Clinical characteristics and genome epidemiology of Stenotrophomonas
maltophilia in Japan. J Antimicrob Chemother. 2024. 79(8):1843-1855.
41
42
43
150. Sakoh T, et al. Cefiderocol susceptibility of 146 Stenotrophomonas maltophilia strains
clinically isolated from blood in two Japanese hospitals over a 10-year period. Eur J Clin
Microbiol Infect Dis. 2024. 43(12):2485-2488.
97