よむ、つかう、まなぶ。
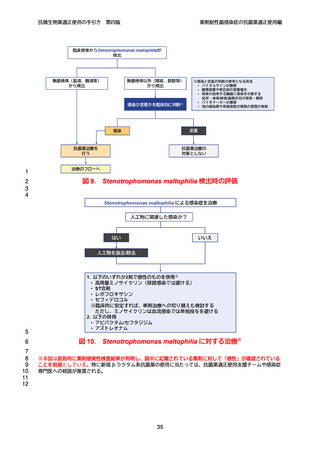
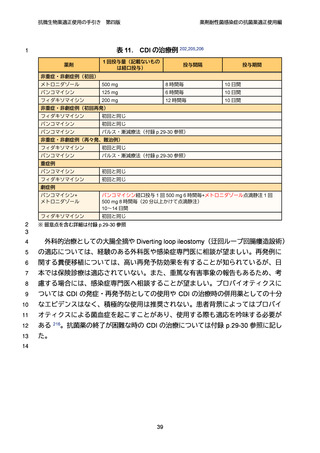
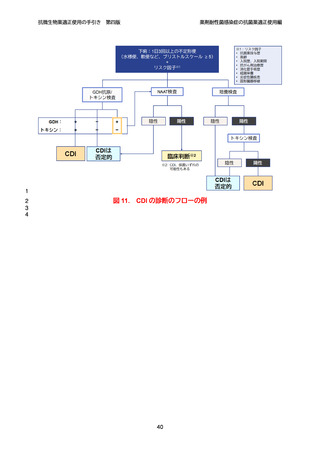
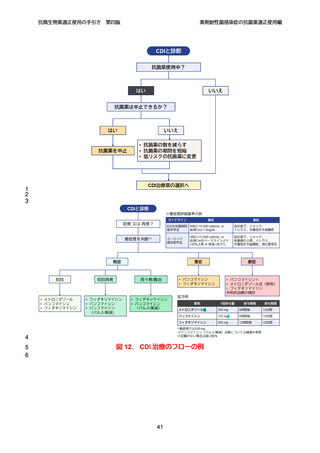
【参考資料2-3】抗微生物薬適正使用の手引き 第四版(案)薬剤耐性菌感染症の抗菌薬適正使用編 (51 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64503.html |
| 出典情報 | 厚生科学審議会 感染症部会(第99回 10/21)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
抗微生物薬適正使用の手引き
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
1
2
3
81. Hsu W, et al. Ceftazidime-avibactam combination therapy versus monotherapy for
treating carbapenem-resistant gram-negative infection: a systemic review and metaanalysis. Infection. 2024. 52(5):2029-2042.
4
5
6
7
82. Paul M, et al. European Society of Clinical Microbiology and Infectious Diseases
(ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gramnegative bacilli (endorsed by European society of intensive care medicine). Clin
Microbiol Infect. 2022. 28(4):521-547.
8
9
10
83. Kayama S, et al. In vitro activity of cefiderocol against carbapenemase-producing and
meropenem-non-susceptible Gram-negative bacteria collected in the Japan
Antimicrobial Resistant Bacterial Surveillance. J Glob Antimicrob Resist. 2024. 38:12-20.
11
12
13
14
84. Gupta N, et al. Ceftazidime-avibactam and aztreonam combination for Carbapenemresistant Enterobacterales bloodstream infections with presumed Metallo-β-lactamase
production: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2024.
22(4):203-209.
15
16
17
85. Timsit JF, et al. Cefiderocol for the Treatment of Infections Due to Metallo-B-lactamaseProducing Pathogens in the CREDIBLE-CR and APEKS-NP Phase 3 Randomized
Studies. Clin Infect Dis. 75(6):1081-1084.
18
19
20
86. Falcone M, et al. Clinical Features and Outcomes of Infections Caused by Metallo-βLactamase-Producing Enterobacterales: A 3-Year Prospective Study From an Endemic
Area. Clin Infect Dis. 2024. 78(5):1111-1119.
21
22
23
87. Lutgring JD, et al. Antibiotic Susceptibility of NDM-Producing Enterobacterales Collected
in the United States in 2017 and 2018. Antimicrob Agents Chemother. 2020.
64(9):e00499-20.
24
25
26
88. Suzuki D, et al. Clinical and genomic characteristics of IMP-producing Enterobacter
cloacae complex and Klebsiella pneumoniae. Antimicrob Agents Chemother. 2024.
68(5):e0167223.
27
28
29
30
89. Ikenoue C, e al. The importance of meropenem resistance, rather than imipenem
resistance, in defining carbapenem-resistant Enterobacterales for public health
surveillance: an analysis of national population-based surveillance. BMC Infect Dis.
2024. 24(1):209.
31
32
33
34
35
36
90. Tsuji BT, et al. International Consensus Guidelines for the Optimal Use of the
Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European
Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases
Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP),
Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases
Pharmacists (SIDP). Pharmacotherapy. 2019. 39:10-39.
37
38
39
91. Zha L, et al. Effectiveness and Safety of High Dose Tigecycline for the Treatment of
Severe Infections: A Systematic Review and Meta-Analysis. Adv Ther. 2020. 37(3):10491064.
40
41
92. De Pascale G, et al. Pharmacokinetics of high-dose tigecycline in critically ill patients
with severe infections. Ann Intensive Care. 2020. 10(1):94.
42
43
44
93. Paul M, et al. Colistin alone versus colistin plus meropenem for treatment of severe
infections caused by carbapenem-resistant Gram-negative bacteria: an open-label,
randomised controlled trial. Lancet Infect Dis. 2018. 18(4):391-400.
45
46
94. Pascale R, et al. Use of meropenem in treating carbapenem-resistant
Enterobacteriaceae infections. Expert Rev Anti Infect Ther. 2019. 17(10):819-827.
51
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
1
2
3
81. Hsu W, et al. Ceftazidime-avibactam combination therapy versus monotherapy for
treating carbapenem-resistant gram-negative infection: a systemic review and metaanalysis. Infection. 2024. 52(5):2029-2042.
4
5
6
7
82. Paul M, et al. European Society of Clinical Microbiology and Infectious Diseases
(ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gramnegative bacilli (endorsed by European society of intensive care medicine). Clin
Microbiol Infect. 2022. 28(4):521-547.
8
9
10
83. Kayama S, et al. In vitro activity of cefiderocol against carbapenemase-producing and
meropenem-non-susceptible Gram-negative bacteria collected in the Japan
Antimicrobial Resistant Bacterial Surveillance. J Glob Antimicrob Resist. 2024. 38:12-20.
11
12
13
14
84. Gupta N, et al. Ceftazidime-avibactam and aztreonam combination for Carbapenemresistant Enterobacterales bloodstream infections with presumed Metallo-β-lactamase
production: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2024.
22(4):203-209.
15
16
17
85. Timsit JF, et al. Cefiderocol for the Treatment of Infections Due to Metallo-B-lactamaseProducing Pathogens in the CREDIBLE-CR and APEKS-NP Phase 3 Randomized
Studies. Clin Infect Dis. 75(6):1081-1084.
18
19
20
86. Falcone M, et al. Clinical Features and Outcomes of Infections Caused by Metallo-βLactamase-Producing Enterobacterales: A 3-Year Prospective Study From an Endemic
Area. Clin Infect Dis. 2024. 78(5):1111-1119.
21
22
23
87. Lutgring JD, et al. Antibiotic Susceptibility of NDM-Producing Enterobacterales Collected
in the United States in 2017 and 2018. Antimicrob Agents Chemother. 2020.
64(9):e00499-20.
24
25
26
88. Suzuki D, et al. Clinical and genomic characteristics of IMP-producing Enterobacter
cloacae complex and Klebsiella pneumoniae. Antimicrob Agents Chemother. 2024.
68(5):e0167223.
27
28
29
30
89. Ikenoue C, e al. The importance of meropenem resistance, rather than imipenem
resistance, in defining carbapenem-resistant Enterobacterales for public health
surveillance: an analysis of national population-based surveillance. BMC Infect Dis.
2024. 24(1):209.
31
32
33
34
35
36
90. Tsuji BT, et al. International Consensus Guidelines for the Optimal Use of the
Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European
Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases
Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP),
Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases
Pharmacists (SIDP). Pharmacotherapy. 2019. 39:10-39.
37
38
39
91. Zha L, et al. Effectiveness and Safety of High Dose Tigecycline for the Treatment of
Severe Infections: A Systematic Review and Meta-Analysis. Adv Ther. 2020. 37(3):10491064.
40
41
92. De Pascale G, et al. Pharmacokinetics of high-dose tigecycline in critically ill patients
with severe infections. Ann Intensive Care. 2020. 10(1):94.
42
43
44
93. Paul M, et al. Colistin alone versus colistin plus meropenem for treatment of severe
infections caused by carbapenem-resistant Gram-negative bacteria: an open-label,
randomised controlled trial. Lancet Infect Dis. 2018. 18(4):391-400.
45
46
94. Pascale R, et al. Use of meropenem in treating carbapenem-resistant
Enterobacteriaceae infections. Expert Rev Anti Infect Ther. 2019. 17(10):819-827.
51