よむ、つかう、まなぶ。
【参考資料2-3】抗微生物薬適正使用の手引き 第四版(案)薬剤耐性菌感染症の抗菌薬適正使用編 (91 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64503.html |
| 出典情報 | 厚生科学審議会 感染症部会(第99回 10/21)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
抗微生物薬適正使用の手引き
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
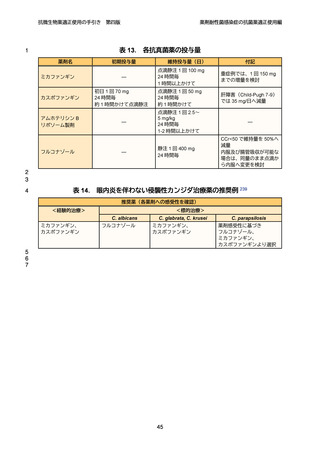
1
2
3
4
45. Kaye KS, et al. Comparison of Treatment Outcomes between Analysis Populations in
the RESTORE-IMI 1 Phase 3 Trial of Imipenem-Cilastatin-Relebactam versus Colistin
plus Imipenem-Cilastatin in Patients with Imipenem-Nonsusceptible Bacterial Infections.
Antimicrob Agents Chemother. 2020. 64(5):e02203-19.
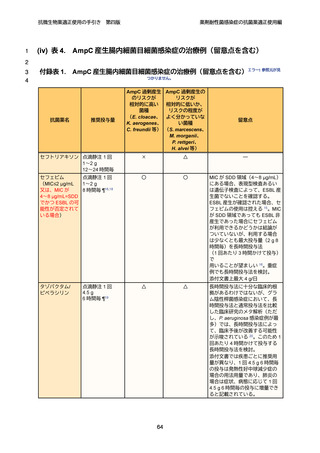
5
6
7
46. Wunderink RG, et al. Cefiderocol versus high-dose, extended-infusion meropenem for
the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised,
double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2021. 21(2):213-225.
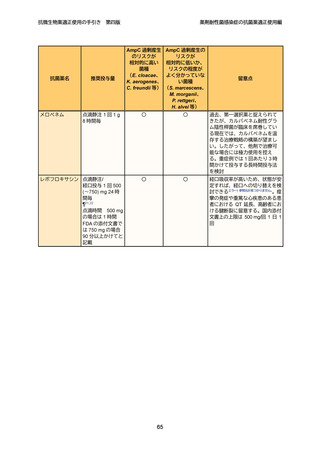
8
9
10
11
12
47. Castanheira M, et al. In Vitro Activity of Plazomicin against Gram-Negative and GramPositive Isolates Collected from U.S. Hospitals and Comparative Activities of
Aminoglycosides against Carbapenem-Resistant Enterobacteriaceae and Isolates
Carrying Carbapenemase Genes. Antimicrob Agents Chemother. 2018. 62(8):e0031318.
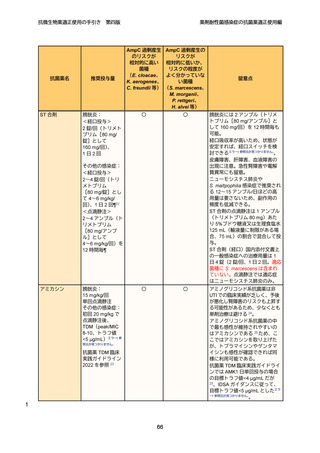
13
14
15
48. Vardakas KZ, et al. Colistin versus polymyxin B for the treatment of patients with
multidrug-resistant Gram-negative infections: a systematic review and meta-analysis. Int
J Antimicrob Agents. 2017. 49(2):233-238.
16
17
18
19
20
21
49. Tsuji BT, et al. International Consensus Guidelines for the Optimal Use of the
Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European
Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases
Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP),
Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases
Pharmacists (SIDP). Pharmacotherapy. 2019. 39(1):10-39.
22
50. Falagas ME, et al. Fosfomycin. Clin Microbiol Rev. 2016 Apr;29(2):321-347.
23
24
25
51. Sojo-Dorado J, et al. Effectiveness of Fosfomycin for the Treatment of MultidrugResistant Escherichia coli Bacteremic Urinary Tract Infections: A Randomized Clinical
Trial. JAMA Netw Open. 2022. 5(1):e2137277.
26
27
28
52. Zha L, et al. Effectiveness and Safety of High Dose Tigecycline for the Treatment of
Severe Infections: A Systematic Review and Meta-Analysis. Adv Ther. 2020. 37(3):10491064.
29
30
53. De Pascale G, et al. Pharmacokinetics of high-dose tigecycline in critically ill patients
with severe infections. Ann Intensive Care. 2020. 10(1):94.
31
32
33
54. Ni W, et al. Tigecycline Treatment for Carbapenem-Resistant Enterobacteriaceae
Infections: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2016.
95(11):e3126.
34
35
55. Pascale R, et al. Use of meropenem in treating carbapenem-resistant
Enterobacteriaceae infections. Expert Rev Anti Infect Ther. 2019. 17(10):819-827.
36
37
38
56. Kayama S, et al. In vitro activity of cefiderocol against carbapenemase-producing and
meropenem-non-susceptible Gram-negative bacteria collected in the Japan
Antimicrobial Resistant Bacterial Surveillance. J Glob Antimicrob Resist. 2024. 38:12-20.
39
40
41
57. Vinks AA, et al. Pharmacokinetics of aztreonam in healthy subjects and patients with
cystic fibrosis and evaluation of dose-exposure relationships using monte carlo
simulation. Antimicrob Agents Chemother. 2007. 51(9):3049-3055.
42
43
44
58. Biagi M, et al. Aztreonam in combination with imipenem-relebactam against clinical and
isogenic strains of serine and metallo-β-lactamase-producing enterobacterales. Diagn
Microbiol Infect Dis. 2022. 103:115674.
91
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
1
2
3
4
45. Kaye KS, et al. Comparison of Treatment Outcomes between Analysis Populations in
the RESTORE-IMI 1 Phase 3 Trial of Imipenem-Cilastatin-Relebactam versus Colistin
plus Imipenem-Cilastatin in Patients with Imipenem-Nonsusceptible Bacterial Infections.
Antimicrob Agents Chemother. 2020. 64(5):e02203-19.
5
6
7
46. Wunderink RG, et al. Cefiderocol versus high-dose, extended-infusion meropenem for
the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised,
double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2021. 21(2):213-225.
8
9
10
11
12
47. Castanheira M, et al. In Vitro Activity of Plazomicin against Gram-Negative and GramPositive Isolates Collected from U.S. Hospitals and Comparative Activities of
Aminoglycosides against Carbapenem-Resistant Enterobacteriaceae and Isolates
Carrying Carbapenemase Genes. Antimicrob Agents Chemother. 2018. 62(8):e0031318.
13
14
15
48. Vardakas KZ, et al. Colistin versus polymyxin B for the treatment of patients with
multidrug-resistant Gram-negative infections: a systematic review and meta-analysis. Int
J Antimicrob Agents. 2017. 49(2):233-238.
16
17
18
19
20
21
49. Tsuji BT, et al. International Consensus Guidelines for the Optimal Use of the
Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European
Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases
Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP),
Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases
Pharmacists (SIDP). Pharmacotherapy. 2019. 39(1):10-39.
22
50. Falagas ME, et al. Fosfomycin. Clin Microbiol Rev. 2016 Apr;29(2):321-347.
23
24
25
51. Sojo-Dorado J, et al. Effectiveness of Fosfomycin for the Treatment of MultidrugResistant Escherichia coli Bacteremic Urinary Tract Infections: A Randomized Clinical
Trial. JAMA Netw Open. 2022. 5(1):e2137277.
26
27
28
52. Zha L, et al. Effectiveness and Safety of High Dose Tigecycline for the Treatment of
Severe Infections: A Systematic Review and Meta-Analysis. Adv Ther. 2020. 37(3):10491064.
29
30
53. De Pascale G, et al. Pharmacokinetics of high-dose tigecycline in critically ill patients
with severe infections. Ann Intensive Care. 2020. 10(1):94.
31
32
33
54. Ni W, et al. Tigecycline Treatment for Carbapenem-Resistant Enterobacteriaceae
Infections: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2016.
95(11):e3126.
34
35
55. Pascale R, et al. Use of meropenem in treating carbapenem-resistant
Enterobacteriaceae infections. Expert Rev Anti Infect Ther. 2019. 17(10):819-827.
36
37
38
56. Kayama S, et al. In vitro activity of cefiderocol against carbapenemase-producing and
meropenem-non-susceptible Gram-negative bacteria collected in the Japan
Antimicrobial Resistant Bacterial Surveillance. J Glob Antimicrob Resist. 2024. 38:12-20.
39
40
41
57. Vinks AA, et al. Pharmacokinetics of aztreonam in healthy subjects and patients with
cystic fibrosis and evaluation of dose-exposure relationships using monte carlo
simulation. Antimicrob Agents Chemother. 2007. 51(9):3049-3055.
42
43
44
58. Biagi M, et al. Aztreonam in combination with imipenem-relebactam against clinical and
isogenic strains of serine and metallo-β-lactamase-producing enterobacterales. Diagn
Microbiol Infect Dis. 2022. 103:115674.
91