よむ、つかう、まなぶ。
【参考資料2-3】抗微生物薬適正使用の手引き 第四版(案)薬剤耐性菌感染症の抗菌薬適正使用編 (57 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64503.html |
| 出典情報 | 厚生科学審議会 感染症部会(第99回 10/21)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
抗微生物薬適正使用の手引き
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
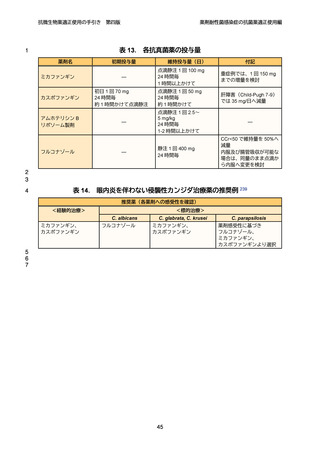
1
2
3
4
179. Lasko MJ, et al. In Vitro Time-Kill Studies of Trimethoprim/Sulfamethoxazole against
Stenotrophomonas maltophilia versus Escherichia coli Using Cation-Adjusted MuellerHinton Broth and ISO-Sensitest Broth. Antimicrob Agents Chemother. 2022.
66(3):e0216721.
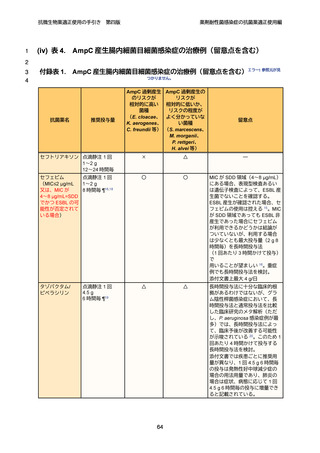
5
6
7
180. Lasko MJ, et al. Trimethoprim/sulfamethoxazole pharmacodynamics against
Stenotrophomonas maltophilia in the in vitro chemostat model. J Antimicrob Chemother.
2022. 77(11):3187-3193.
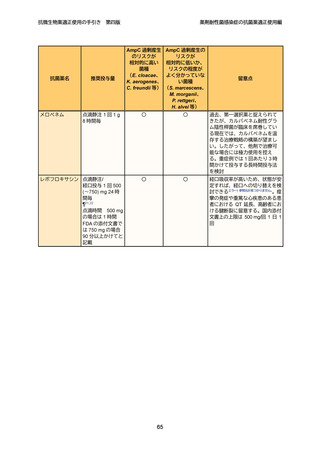
8
9
181. Mojica MF, et al. Treatment approaches for severe Stenotrophomonas maltophilia
infections. Curr Opin Infect Dis. 2023. 36(6):572-584.
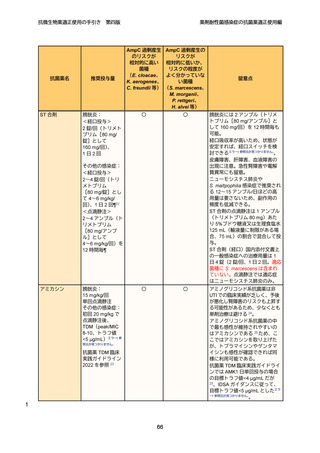
10
11
12
182. Nys C, et al. Clinical and Microbiologic Outcomes in Patients with Monomicrobial
Stenotrophomonas maltophilia Infections. Antimicrob Agents Chemother. 2019.
63(11):e00788-19.
13
14
15
16
183. Hackel MA, et al In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against
a Recent Collection of Clinically Relevant Gram-Negative Bacilli from North America and
Europe, Including Carbapenem-Nonsusceptible Isolates (SIDERO-WT-2014 Study).
Antimicrob Agents Chemother. 2017. 61(9):e00093-17.
17
18
19
184. Sakoh T, et al. Cefiderocol susceptibility of 146 Stenotrophomonas maltophilia strains
clinically isolated from blood in two Japanese hospitals over a 10-year period. Eur J Clin
Microbiol Infect Dis. 2024. 43(12):2485-2488.
20
21
22
185. Shah MD, et al. Efficacy of combination therapy versus monotherapy in the treatment of
Stenotrophomonas maltophilia pneumonia. J Antimicrob Chemother. 2019. 74(7):20552059.
23
24
186. Leffler DA, et al.Clostridium difficile infection. N Engl J Med. 2015 Apr 16;372(16):153948.
25
26
27
187. Magill SS, et al. Emerging Infections Program Hospital Prevalence Survey Team.
Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N Engl J
Med. 2018. 379(18):1732-1744.
28
29
188. Marra AR, et al. Incidence and Outcomes Associated With Clostridium difficile Infections:
A Systematic Review and Meta-analysis. JAMA Netw Open. 2020. 3(1):e1917597.
30
31
189. Kato H, et al. Clostridioides (Clostridium) difficile infection burden in Japan: A multicenter
prospective study. Anaerobe. 2019. 60:102011.
32
33
190. Kordus SL, et al. Clostridioides difficile toxins: mechanisms of action and antitoxin
therapeutics. Nat Rev Microbiol. 2022. 20(5):285-298.
34
35
191. Eckert C, et al. Prevalence and pathogenicity of binary toxin-positive Clostridium difficile
strains that do not produce toxins A and B. New Microbes New Infect. 2014. 3:12-7.
36
37
192. Crobach MJT, et al. Understanding Clostridium difficile Colonization. Clin Microbiol Rev.
2018. 31(2):e00021-17.
38
39
40
193. Warny M, et al. Toxin production by an emerging strain of Clostridium difficile associated
with outbreaks of severe disease in North America and Europe. Lancet. 2005.
30;366(9491):1079-84.
41
42
194. Abou Chakra CN, et al. The Strain and the Clinical Outcome of Clostridioides
difficile Infection: A Meta-analysis. Open Forum Infect Dis. 2024. 11(3):ofae085.
43
44
45
195. Iwashima Y, et al. A retrospective study of the epidemiology of Clostridium difficile
infection at a University Hospital in Japan: genotypic features of the isolates and clinical
characteristics of the patients. J Infect Chemother. 2010. 16(5):329-33.
57
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
1
2
3
4
179. Lasko MJ, et al. In Vitro Time-Kill Studies of Trimethoprim/Sulfamethoxazole against
Stenotrophomonas maltophilia versus Escherichia coli Using Cation-Adjusted MuellerHinton Broth and ISO-Sensitest Broth. Antimicrob Agents Chemother. 2022.
66(3):e0216721.
5
6
7
180. Lasko MJ, et al. Trimethoprim/sulfamethoxazole pharmacodynamics against
Stenotrophomonas maltophilia in the in vitro chemostat model. J Antimicrob Chemother.
2022. 77(11):3187-3193.
8
9
181. Mojica MF, et al. Treatment approaches for severe Stenotrophomonas maltophilia
infections. Curr Opin Infect Dis. 2023. 36(6):572-584.
10
11
12
182. Nys C, et al. Clinical and Microbiologic Outcomes in Patients with Monomicrobial
Stenotrophomonas maltophilia Infections. Antimicrob Agents Chemother. 2019.
63(11):e00788-19.
13
14
15
16
183. Hackel MA, et al In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against
a Recent Collection of Clinically Relevant Gram-Negative Bacilli from North America and
Europe, Including Carbapenem-Nonsusceptible Isolates (SIDERO-WT-2014 Study).
Antimicrob Agents Chemother. 2017. 61(9):e00093-17.
17
18
19
184. Sakoh T, et al. Cefiderocol susceptibility of 146 Stenotrophomonas maltophilia strains
clinically isolated from blood in two Japanese hospitals over a 10-year period. Eur J Clin
Microbiol Infect Dis. 2024. 43(12):2485-2488.
20
21
22
185. Shah MD, et al. Efficacy of combination therapy versus monotherapy in the treatment of
Stenotrophomonas maltophilia pneumonia. J Antimicrob Chemother. 2019. 74(7):20552059.
23
24
186. Leffler DA, et al.Clostridium difficile infection. N Engl J Med. 2015 Apr 16;372(16):153948.
25
26
27
187. Magill SS, et al. Emerging Infections Program Hospital Prevalence Survey Team.
Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N Engl J
Med. 2018. 379(18):1732-1744.
28
29
188. Marra AR, et al. Incidence and Outcomes Associated With Clostridium difficile Infections:
A Systematic Review and Meta-analysis. JAMA Netw Open. 2020. 3(1):e1917597.
30
31
189. Kato H, et al. Clostridioides (Clostridium) difficile infection burden in Japan: A multicenter
prospective study. Anaerobe. 2019. 60:102011.
32
33
190. Kordus SL, et al. Clostridioides difficile toxins: mechanisms of action and antitoxin
therapeutics. Nat Rev Microbiol. 2022. 20(5):285-298.
34
35
191. Eckert C, et al. Prevalence and pathogenicity of binary toxin-positive Clostridium difficile
strains that do not produce toxins A and B. New Microbes New Infect. 2014. 3:12-7.
36
37
192. Crobach MJT, et al. Understanding Clostridium difficile Colonization. Clin Microbiol Rev.
2018. 31(2):e00021-17.
38
39
40
193. Warny M, et al. Toxin production by an emerging strain of Clostridium difficile associated
with outbreaks of severe disease in North America and Europe. Lancet. 2005.
30;366(9491):1079-84.
41
42
194. Abou Chakra CN, et al. The Strain and the Clinical Outcome of Clostridioides
difficile Infection: A Meta-analysis. Open Forum Infect Dis. 2024. 11(3):ofae085.
43
44
45
195. Iwashima Y, et al. A retrospective study of the epidemiology of Clostridium difficile
infection at a University Hospital in Japan: genotypic features of the isolates and clinical
characteristics of the patients. J Infect Chemother. 2010. 16(5):329-33.
57