よむ、つかう、まなぶ。
【参考資料2-3】抗微生物薬適正使用の手引き 第四版(案)薬剤耐性菌感染症の抗菌薬適正使用編 (58 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64503.html |
| 出典情報 | 厚生科学審議会 感染症部会(第99回 10/21)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
抗微生物薬適正使用の手引き
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
1
2
3
196. Di Bella S, et al. Clostridioides difficile infection: history, epidemiology, risk factors,
prevention, clinical manifestations, treatment, and future options. Clin Microbiol Rev.
2024. 13;37(2):e0013523.
4
5
197. Manzoor F, et al. Does this patient have Clostridioides difficile infection? A systematic
review and meta-analysis. Clin Microbiol Infect. 2023. 29(11):1367-1374.
6
7
198. Mattila E, et al. Extraintestinal Clostridium difficile infections. Clin Infect Dis. 2013.
57(6):e148-53.
8
9
199. Keessen EC, et al. Antimicrobial susceptibility profiles of human and piglet Clostridium
difficile PCR-ribotype 078. Antimicrob Resist Infect Control. 2013. 2:14.
10
11
12
200. Privitera G, et al. Prospective study of Clostridium difficile intestinal colonization and
disease following single-dose antibiotic prophylaxis in surgery. Antimicrob Agents
Chemother. 1991. 35(1):208-210.
13
14
15
201. Guh AY, et al. Emerging Infections Program Clostridioides difficile Infection Working
Group. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N Engl J
Med. 2020. 382(14):1320-1330.
16
17
18
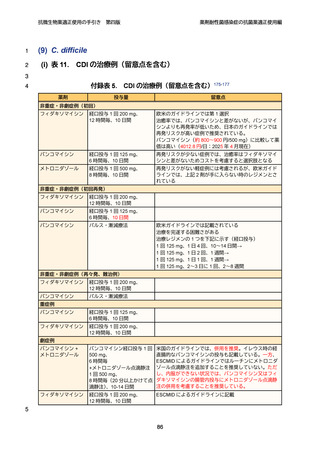
202. 公益社団法人日本化学療法学会・一般社団法人日本感染症学会 CDI 診療ガイドライン
作成委員会編. Clostridioides difficile 感染症診療ガイドライン. 2022. at
https://www.kansensho.or.jp/uploads/files/guidelines/guideline_cdi_230125.pdf.)
19
20
203. Kociolek LK, et al. Strategies to prevent Clostridioides difficile infections in acute-care
hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2023. 44(4):527-549.
21
22
204. Finn E, et al. Burden of Clostridioides difficile infection (CDI) - a systematic review of the
epidemiology of primary and recurrent CDI. BMC Infect Dis. 2021. 21(1):456.
23
24
25
205. van Prehn J, et al. European Society of Clinical Microbiology and Infectious Diseases:
2021 update on the treatment guidance document for Clostridioides difficile infection in
adults. Clin Microbiol Infect. 2021. 27 Suppl 2:S1-S21.
26
27
28
29
206. Johnson S, et al. Clinical Practice Guideline by the Infectious Diseases Society of
America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021
Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults.
Clin Infect Dis. 2021. 73(5):755-757.
30
31
32
207. Figueroa I, et al. Relapse versus reinfection: recurrent Clostridium difficile infection
following treatment with fidaxomicin or vancomycin. Clin Infect Dis. 2012. 55 Suppl
2:S104-9.
33
34
208. Johnson S. Recurrent Clostridium difficile infection: causality and therapeutic
approaches. Int J Antimicrob Agents. 2009. 33 Suppl 1:S33-6.
35
36
209. Pepin J, et al. Management and outcomes of a first recurrence of Clostridium difficileassociated disease in Quebec, Canada. Clin Infect Dis. 2006. 42(6):758-764.
37
38
210. McFarland LV, et al. Breaking the cycle: treatment strategies for 163 cases of recurrent
Clostridium difficile disease. Am J Gastroenterol. 2002. 97(7):1769-1775.
39
40
211. Kociolek LK, et al. Strategies to prevent Clostridioides difficile infections in acute-care
hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2023. 44(4):527-549.
41
42
212. Polage CR, et al. Overdiagnosis of Clostridium difficile Infection in the Molecular Test
Era. JAMA Intern Med. 2015. 175(11):1792-1801.
43
44
45
213. Okumura H, et al. Fidaxomicin compared with vancomycin and metronidazole for the
treatment of Clostridioides (Clostridium) difficile infection: A network meta-analysis. J
Infect Chemother. 2020. 26(1):43-50.
58
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
1
2
3
196. Di Bella S, et al. Clostridioides difficile infection: history, epidemiology, risk factors,
prevention, clinical manifestations, treatment, and future options. Clin Microbiol Rev.
2024. 13;37(2):e0013523.
4
5
197. Manzoor F, et al. Does this patient have Clostridioides difficile infection? A systematic
review and meta-analysis. Clin Microbiol Infect. 2023. 29(11):1367-1374.
6
7
198. Mattila E, et al. Extraintestinal Clostridium difficile infections. Clin Infect Dis. 2013.
57(6):e148-53.
8
9
199. Keessen EC, et al. Antimicrobial susceptibility profiles of human and piglet Clostridium
difficile PCR-ribotype 078. Antimicrob Resist Infect Control. 2013. 2:14.
10
11
12
200. Privitera G, et al. Prospective study of Clostridium difficile intestinal colonization and
disease following single-dose antibiotic prophylaxis in surgery. Antimicrob Agents
Chemother. 1991. 35(1):208-210.
13
14
15
201. Guh AY, et al. Emerging Infections Program Clostridioides difficile Infection Working
Group. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N Engl J
Med. 2020. 382(14):1320-1330.
16
17
18
202. 公益社団法人日本化学療法学会・一般社団法人日本感染症学会 CDI 診療ガイドライン
作成委員会編. Clostridioides difficile 感染症診療ガイドライン. 2022. at
https://www.kansensho.or.jp/uploads/files/guidelines/guideline_cdi_230125.pdf.)
19
20
203. Kociolek LK, et al. Strategies to prevent Clostridioides difficile infections in acute-care
hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2023. 44(4):527-549.
21
22
204. Finn E, et al. Burden of Clostridioides difficile infection (CDI) - a systematic review of the
epidemiology of primary and recurrent CDI. BMC Infect Dis. 2021. 21(1):456.
23
24
25
205. van Prehn J, et al. European Society of Clinical Microbiology and Infectious Diseases:
2021 update on the treatment guidance document for Clostridioides difficile infection in
adults. Clin Microbiol Infect. 2021. 27 Suppl 2:S1-S21.
26
27
28
29
206. Johnson S, et al. Clinical Practice Guideline by the Infectious Diseases Society of
America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021
Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults.
Clin Infect Dis. 2021. 73(5):755-757.
30
31
32
207. Figueroa I, et al. Relapse versus reinfection: recurrent Clostridium difficile infection
following treatment with fidaxomicin or vancomycin. Clin Infect Dis. 2012. 55 Suppl
2:S104-9.
33
34
208. Johnson S. Recurrent Clostridium difficile infection: causality and therapeutic
approaches. Int J Antimicrob Agents. 2009. 33 Suppl 1:S33-6.
35
36
209. Pepin J, et al. Management and outcomes of a first recurrence of Clostridium difficileassociated disease in Quebec, Canada. Clin Infect Dis. 2006. 42(6):758-764.
37
38
210. McFarland LV, et al. Breaking the cycle: treatment strategies for 163 cases of recurrent
Clostridium difficile disease. Am J Gastroenterol. 2002. 97(7):1769-1775.
39
40
211. Kociolek LK, et al. Strategies to prevent Clostridioides difficile infections in acute-care
hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2023. 44(4):527-549.
41
42
212. Polage CR, et al. Overdiagnosis of Clostridium difficile Infection in the Molecular Test
Era. JAMA Intern Med. 2015. 175(11):1792-1801.
43
44
45
213. Okumura H, et al. Fidaxomicin compared with vancomycin and metronidazole for the
treatment of Clostridioides (Clostridium) difficile infection: A network meta-analysis. J
Infect Chemother. 2020. 26(1):43-50.
58