よむ、つかう、まなぶ。
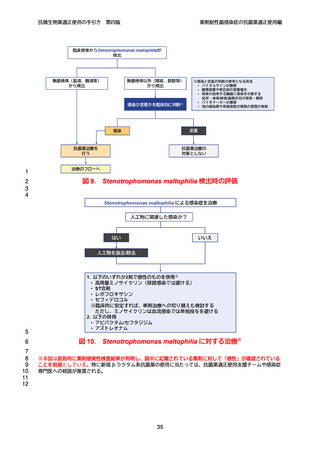
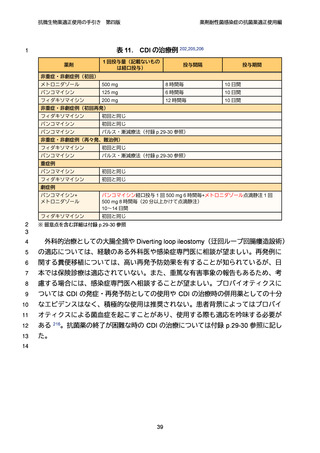
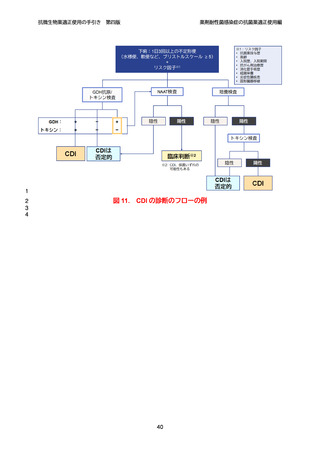
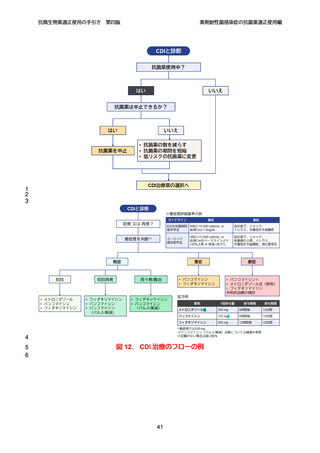
【参考資料2-3】抗微生物薬適正使用の手引き 第四版(案)薬剤耐性菌感染症の抗菌薬適正使用編 (52 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64503.html |
| 出典情報 | 厚生科学審議会 感染症部会(第99回 10/21)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
抗微生物薬適正使用の手引き
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
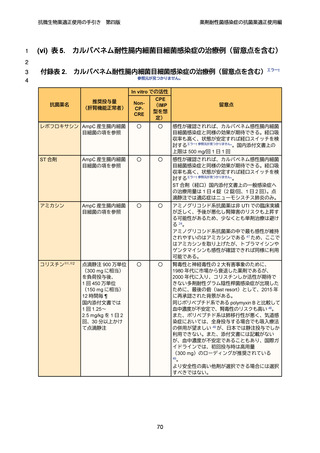
1
2
3
95. Vinks AA, et al. Pharmacokinetics of aztreonam in healthy subjects and patients with
cystic fibrosis and evaluation of dose-exposure relationships using monte carlo
simulation. Antimicrob Agents Chemother. 2007. 51(9):3049-55.
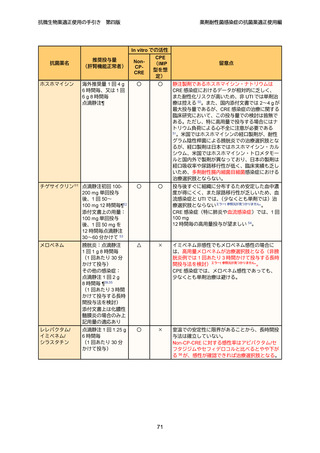
4
5
6
96. Tumbarello M, et al. Ceftazidime-Avibactam Use for Klebsiella pneumoniae
Carbapenemase-Producing K. pneumoniae Infections: A Retrospective Observational
Multicenter Study. Clin Infect Dis. 2021. 73(9):1664-1676.
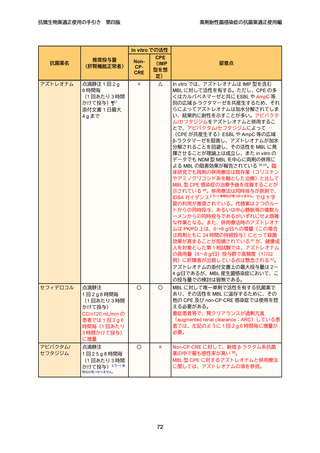
7
8
9
97. コリスチンの適正使用に関する指針―改訂版―. 日本化学療法学会雑誌. 2015.
63(3):290-329. at
https://www.chemotherapy.or.jp/uploads/files/guideline/colistin_guideline_update.pdf.)
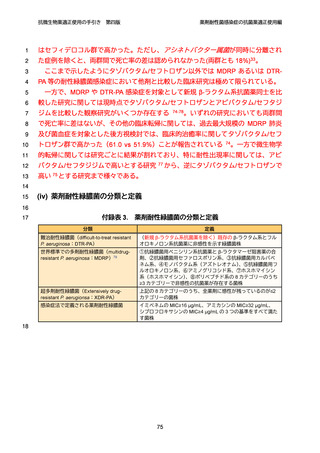
10
98. チゲサイクリン適正使用のための手引き. 日本化学療法学会雑誌. 2014. 62(3):311-366.
11
12
13
99. Tamma PD, et al. Carbapenem therapy is associated with improved survival compared
with piperacillin-tazobactam for patients with extended-spectrum β-lactamase
bacteremia. Clin Infect Dis. 2015. 60(9):1319-1325.
14
15
16
100. NICE Guideline. Sepsis: recognition, diagnosis and early management. 2017. at
https://www.nice.org.uk/guidance/ng51/resources/sepsis-recognition-diagnosis-andearly-management-pdf-1837508256709.)
17
18
19
101. Heil EL, et al. Optimizing the Management of Uncomplicated Gram-Negative
Bloodstream Infections: Consensus Guidance Using a Modified Delphi Process. Open
Forum Infect Dis. 2021. 8(10):ofab434.
20
21
102. Poutsiaka DD, et al. Risk factors for death after sepsis in patients immunosuppressed
before the onset of sepsis. Scand J Infect Dis. 2009. 41(6-7):469-479.
22
23
103. Evans L, et al. Surviving sepsis campaign: international guidelines for management of
sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-1247.
24
25
104. 厚生労働省 薬剤耐性緑膿菌感染症. at https://www.mhlw.go.jp/bunya/kenkou/kekkakukansenshou11/01-05-42-01.html.)
26
27
28
105. Kadri SS, et al. Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US
Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of
Resistance to All First-line Agents. Clin Infect Dis. 2018. 67(12):1803-1814.
29
30
106. Yano H, et al. Nationwide genome surveillance of carbapenem-resistant Pseudomonas
aeruginosa in Japan. Antimicrob Agents Chemother. 2024. 68(5):e0166923.
31
32
33
107. Nakayama R, et al. Classification of the metallo β-lactamase subtype produced by the
carbapenem-resistant Pseudomonas aeruginosa isolates in Japan. J Infect Chemother.
2022. 28(2):170-175.
34
35
36
108. Uechi K, et al. A Modified Carbapenem Inactivation Method, CIMTris, for
Carbapenemase Production in Acinetobacter and Pseudomonas Species. J Clin
Microbiol. 2017. 55(12):3405-3410.
37
38
39
109. Almangour TA, et al. Ceftolozane-tazobactam vs. colistin for the treatment of infections
due to multidrug-resistant Pseudomonas aeruginosa: a multicentre cohort study. J Glob
Antimicrob Resist. 2022. 28:288-294.
40
41
42
110. Pogue JM, et al. Ceftolozane/Tazobactam vs Polymyxin or Aminoglycoside-based
Regimens for the Treatment of Drug-resistant Pseudomonas aeruginosa. Clin Infect Dis.
2020. 71(2):304-310.
43
44
45
46
111. Almangour TA, et al. Effectiveness of ceftazidime-avibactam versus ceftolozanetazobactam for multidrug-resistant Pseudomonas aeruginosa infections in the USA
(CACTUS): a multicentre, retrospective, observational study. Lancet Infect Dis. 2025.
25(5):574-584.
52
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
1
2
3
95. Vinks AA, et al. Pharmacokinetics of aztreonam in healthy subjects and patients with
cystic fibrosis and evaluation of dose-exposure relationships using monte carlo
simulation. Antimicrob Agents Chemother. 2007. 51(9):3049-55.
4
5
6
96. Tumbarello M, et al. Ceftazidime-Avibactam Use for Klebsiella pneumoniae
Carbapenemase-Producing K. pneumoniae Infections: A Retrospective Observational
Multicenter Study. Clin Infect Dis. 2021. 73(9):1664-1676.
7
8
9
97. コリスチンの適正使用に関する指針―改訂版―. 日本化学療法学会雑誌. 2015.
63(3):290-329. at
https://www.chemotherapy.or.jp/uploads/files/guideline/colistin_guideline_update.pdf.)
10
98. チゲサイクリン適正使用のための手引き. 日本化学療法学会雑誌. 2014. 62(3):311-366.
11
12
13
99. Tamma PD, et al. Carbapenem therapy is associated with improved survival compared
with piperacillin-tazobactam for patients with extended-spectrum β-lactamase
bacteremia. Clin Infect Dis. 2015. 60(9):1319-1325.
14
15
16
100. NICE Guideline. Sepsis: recognition, diagnosis and early management. 2017. at
https://www.nice.org.uk/guidance/ng51/resources/sepsis-recognition-diagnosis-andearly-management-pdf-1837508256709.)
17
18
19
101. Heil EL, et al. Optimizing the Management of Uncomplicated Gram-Negative
Bloodstream Infections: Consensus Guidance Using a Modified Delphi Process. Open
Forum Infect Dis. 2021. 8(10):ofab434.
20
21
102. Poutsiaka DD, et al. Risk factors for death after sepsis in patients immunosuppressed
before the onset of sepsis. Scand J Infect Dis. 2009. 41(6-7):469-479.
22
23
103. Evans L, et al. Surviving sepsis campaign: international guidelines for management of
sepsis and septic shock 2021. Intensive Care Med. 2021 Nov;47(11):1181-1247.
24
25
104. 厚生労働省 薬剤耐性緑膿菌感染症. at https://www.mhlw.go.jp/bunya/kenkou/kekkakukansenshou11/01-05-42-01.html.)
26
27
28
105. Kadri SS, et al. Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US
Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of
Resistance to All First-line Agents. Clin Infect Dis. 2018. 67(12):1803-1814.
29
30
106. Yano H, et al. Nationwide genome surveillance of carbapenem-resistant Pseudomonas
aeruginosa in Japan. Antimicrob Agents Chemother. 2024. 68(5):e0166923.
31
32
33
107. Nakayama R, et al. Classification of the metallo β-lactamase subtype produced by the
carbapenem-resistant Pseudomonas aeruginosa isolates in Japan. J Infect Chemother.
2022. 28(2):170-175.
34
35
36
108. Uechi K, et al. A Modified Carbapenem Inactivation Method, CIMTris, for
Carbapenemase Production in Acinetobacter and Pseudomonas Species. J Clin
Microbiol. 2017. 55(12):3405-3410.
37
38
39
109. Almangour TA, et al. Ceftolozane-tazobactam vs. colistin for the treatment of infections
due to multidrug-resistant Pseudomonas aeruginosa: a multicentre cohort study. J Glob
Antimicrob Resist. 2022. 28:288-294.
40
41
42
110. Pogue JM, et al. Ceftolozane/Tazobactam vs Polymyxin or Aminoglycoside-based
Regimens for the Treatment of Drug-resistant Pseudomonas aeruginosa. Clin Infect Dis.
2020. 71(2):304-310.
43
44
45
46
111. Almangour TA, et al. Effectiveness of ceftazidime-avibactam versus ceftolozanetazobactam for multidrug-resistant Pseudomonas aeruginosa infections in the USA
(CACTUS): a multicentre, retrospective, observational study. Lancet Infect Dis. 2025.
25(5):574-584.
52