よむ、つかう、まなぶ。
【参考資料2-3】抗微生物薬適正使用の手引き 第四版(案)薬剤耐性菌感染症の抗菌薬適正使用編 (94 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64503.html |
| 出典情報 | 厚生科学審議会 感染症部会(第99回 10/21)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
抗微生物薬適正使用の手引き
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
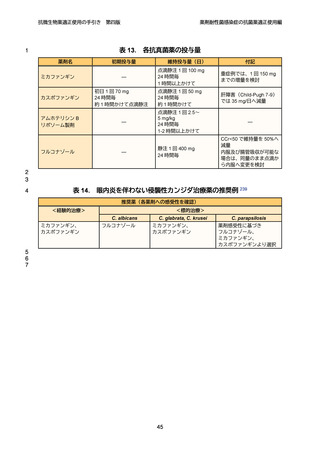
1
2
3
88. Xiao AJ, et al. Ceftolozane/tazobactam pharmacokinetic/pharmacodynamic-derived
dose justification for phase 3 studies in patients with nosocomial pneumonia. J Clin
Pharmacol. 2016. 56(1):56-66.
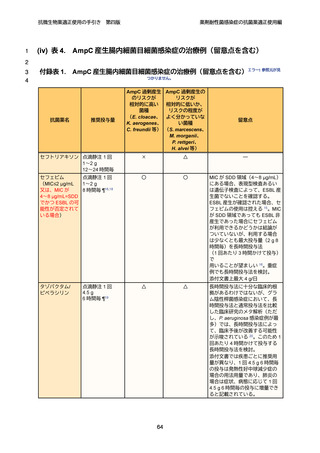
4
5
6
89. Terrier CL, et al. In vitro activity of aztreonam in combination with newly developed βlactamase inhibitors against MDR Enterobacterales and Pseudomonas aeruginosa
producing metallo-β-lactamases. J Antimicrob Chemother. 2022. 78(1):101-107.
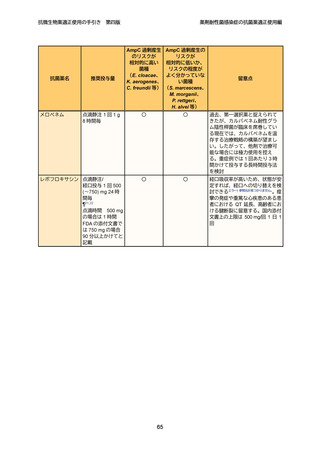
7
8
9
90. Mensa J, et al. Antibiotic selection in the treatment of acute invasive infections by
Pseudomonas aeruginosa: Guidelines by the Spanish Society of Chemotherapy. Rev
Esp Quimioter. 2018. 31(1):78-100.
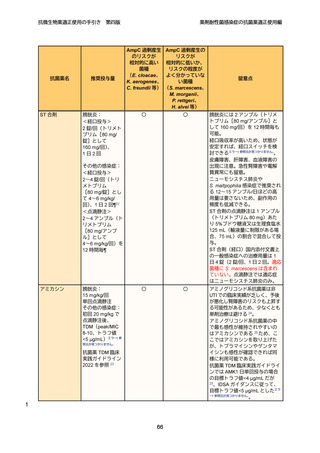
10
11
12
91. Kluge RM, et al. Comparative activity of tobramycin, amikacin, and gentamicin alone and
with carbenicillin against Pseudomonas aeruginosa. Antimicrob Agents Chemother.
1974. 6(4):442-446.
13
14
15
92. GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial
antimicrobial resistance 1990-2021: a systematic analysis with forecasts to 2050.
Lancet. 2024. 404(10459):1199-1226.
16
17
93. Antimicrobial Resistance C. Global burden of bacterial antimicrobial resistance in 2019:
a systematic analysis. Lancet. 2022. 399(10325):629-655.
18
19
20
21
94. Gales AC, et al. Antimicrobial Susceptibility of Acinetobacter calcoaceticus-Acinetobacter
baumannii Complex and Stenotrophomonas maltophilia Clinical Isolates: Results From
the SENTRY Antimicrobial Surveillance Program (1997-2016). Open Forum Infect Dis.
2019. 6(Suppl 1):S34-S46.
22
23
95. Hsu L-Y, et al. Carbapenem-Resistant Acinetobacter baumannii and Enterobacteriaceae
in South and Southeast Asia. Clin Microbiol Rev. 2017. 30(1):1-22.
24
25
26
96. Iovleva A, et al. Carbapenem-Resistant Acinetobacter baumannii in U.S. Hospitals:
Diversification of Circulating Lineages and Antimicrobial Resistance. mBio. 2022.
3(2):e0275921.
27
28
97. Higgins PG, et al. Global spread of carbapenem-resistant Acinetobacter baumannii. J
Antimicrob Chemother. 2010. 65(2):233-238.
29
98. Evans BA, et al. OXA β-lactamases. Clin Microbiol Rev. 2014. 27(2):241-263.
30
31
32
99. Lee CR, et al. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance
Mechanisms, and Prospective Treatment Options. Front Cell Infect Microbiol. 2017.
7:55.
33
34
35
100. Yamamoto M, et al. Interspecies dissemination of a novel class 1 integron carrying
blaIMP-19 among Acinetobacter species in Japan. J Antimicrob Chemother. 2011.
66(11):2480-2483.
36
37
38
101. Castanheira M, Mendes RE, et al. Global Epidemiology and Mechanisms of Resistance
of Acinetobacter baumannii-calcoaceticus Complex. Clin Infect Dis. 2023. 76(Suppl
2):S166-S178.
39
40
102. 薬剤耐性アシネトバクター感染症. at https://www.mhlw.go.jp/bunya/kenkou/kekkakukansenshou11/01-05-140912-4.html.)
41
42
103. 国立感染症研究所 感染症法に基づく薬剤耐性アシネトバクター感染症の届出状況.
2019. at https://www.niid.go.jp/niid/ja/mdra-m/mdra-idwrs/10322-mdra-210423.html.
43
44
104. 厚生労働省 院内感染対策サーベイランス 薬剤耐性菌 判定基準(Ver.3.2). 2019. at
https://janis.mhlw.go.jp/section/standard/drugresistancestandard_ver3.2_20190109.pdf.
94
第四版
薬剤耐性菌感染症の抗菌薬適正使用編
1
2
3
88. Xiao AJ, et al. Ceftolozane/tazobactam pharmacokinetic/pharmacodynamic-derived
dose justification for phase 3 studies in patients with nosocomial pneumonia. J Clin
Pharmacol. 2016. 56(1):56-66.
4
5
6
89. Terrier CL, et al. In vitro activity of aztreonam in combination with newly developed βlactamase inhibitors against MDR Enterobacterales and Pseudomonas aeruginosa
producing metallo-β-lactamases. J Antimicrob Chemother. 2022. 78(1):101-107.
7
8
9
90. Mensa J, et al. Antibiotic selection in the treatment of acute invasive infections by
Pseudomonas aeruginosa: Guidelines by the Spanish Society of Chemotherapy. Rev
Esp Quimioter. 2018. 31(1):78-100.
10
11
12
91. Kluge RM, et al. Comparative activity of tobramycin, amikacin, and gentamicin alone and
with carbenicillin against Pseudomonas aeruginosa. Antimicrob Agents Chemother.
1974. 6(4):442-446.
13
14
15
92. GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial
antimicrobial resistance 1990-2021: a systematic analysis with forecasts to 2050.
Lancet. 2024. 404(10459):1199-1226.
16
17
93. Antimicrobial Resistance C. Global burden of bacterial antimicrobial resistance in 2019:
a systematic analysis. Lancet. 2022. 399(10325):629-655.
18
19
20
21
94. Gales AC, et al. Antimicrobial Susceptibility of Acinetobacter calcoaceticus-Acinetobacter
baumannii Complex and Stenotrophomonas maltophilia Clinical Isolates: Results From
the SENTRY Antimicrobial Surveillance Program (1997-2016). Open Forum Infect Dis.
2019. 6(Suppl 1):S34-S46.
22
23
95. Hsu L-Y, et al. Carbapenem-Resistant Acinetobacter baumannii and Enterobacteriaceae
in South and Southeast Asia. Clin Microbiol Rev. 2017. 30(1):1-22.
24
25
26
96. Iovleva A, et al. Carbapenem-Resistant Acinetobacter baumannii in U.S. Hospitals:
Diversification of Circulating Lineages and Antimicrobial Resistance. mBio. 2022.
3(2):e0275921.
27
28
97. Higgins PG, et al. Global spread of carbapenem-resistant Acinetobacter baumannii. J
Antimicrob Chemother. 2010. 65(2):233-238.
29
98. Evans BA, et al. OXA β-lactamases. Clin Microbiol Rev. 2014. 27(2):241-263.
30
31
32
99. Lee CR, et al. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance
Mechanisms, and Prospective Treatment Options. Front Cell Infect Microbiol. 2017.
7:55.
33
34
35
100. Yamamoto M, et al. Interspecies dissemination of a novel class 1 integron carrying
blaIMP-19 among Acinetobacter species in Japan. J Antimicrob Chemother. 2011.
66(11):2480-2483.
36
37
38
101. Castanheira M, Mendes RE, et al. Global Epidemiology and Mechanisms of Resistance
of Acinetobacter baumannii-calcoaceticus Complex. Clin Infect Dis. 2023. 76(Suppl
2):S166-S178.
39
40
102. 薬剤耐性アシネトバクター感染症. at https://www.mhlw.go.jp/bunya/kenkou/kekkakukansenshou11/01-05-140912-4.html.)
41
42
103. 国立感染症研究所 感染症法に基づく薬剤耐性アシネトバクター感染症の届出状況.
2019. at https://www.niid.go.jp/niid/ja/mdra-m/mdra-idwrs/10322-mdra-210423.html.
43
44
104. 厚生労働省 院内感染対策サーベイランス 薬剤耐性菌 判定基準(Ver.3.2). 2019. at
https://janis.mhlw.go.jp/section/standard/drugresistancestandard_ver3.2_20190109.pdf.
94