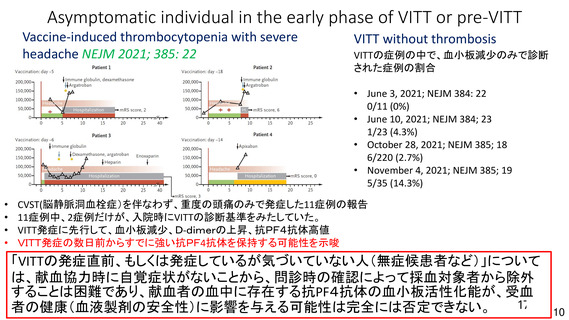
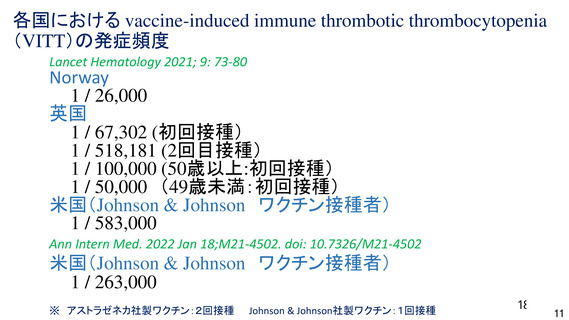
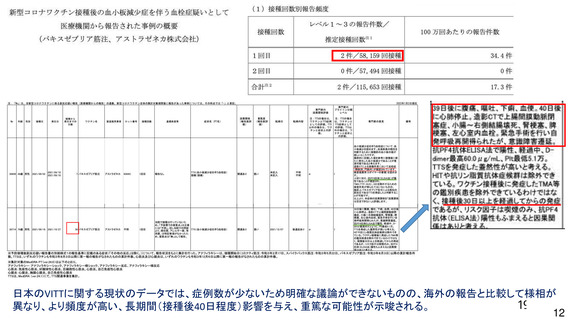
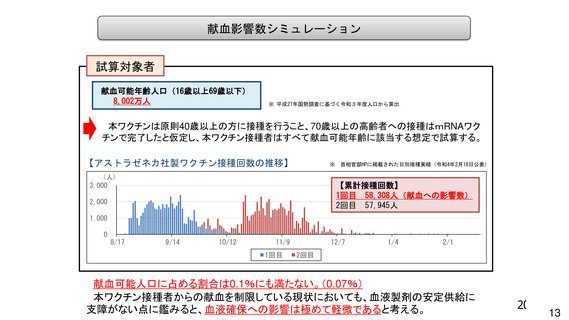
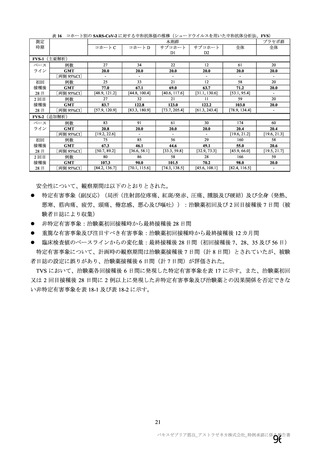
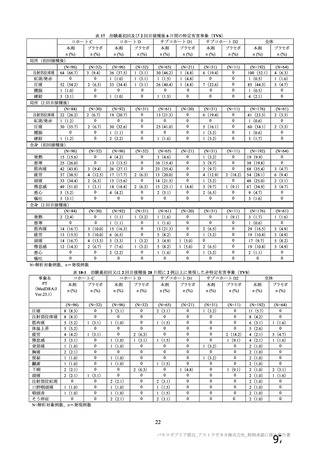
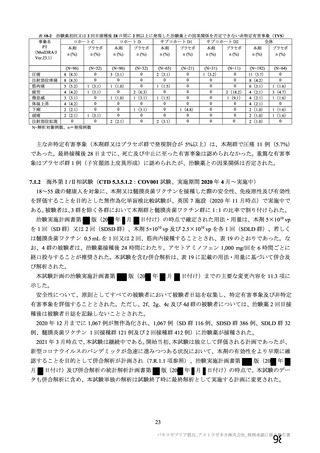
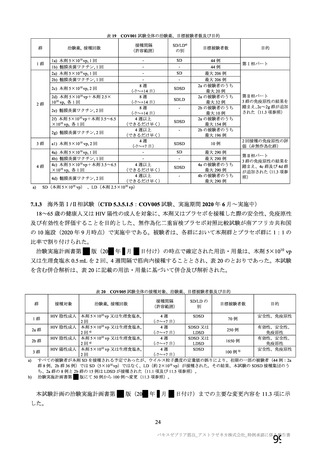
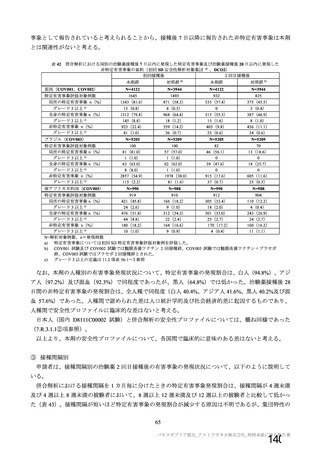
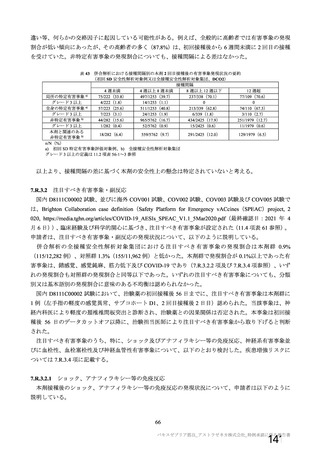
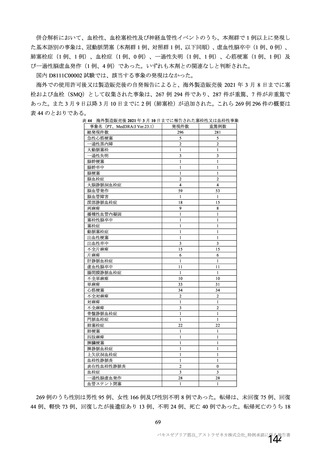
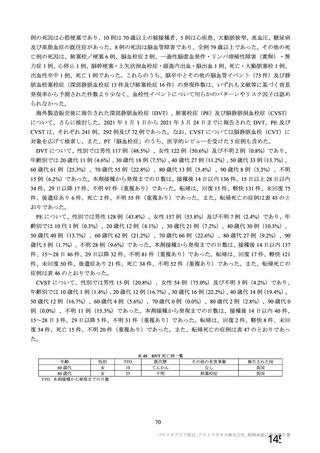
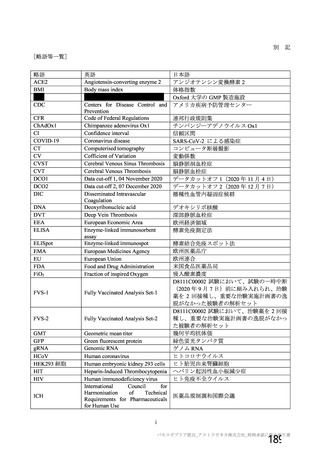
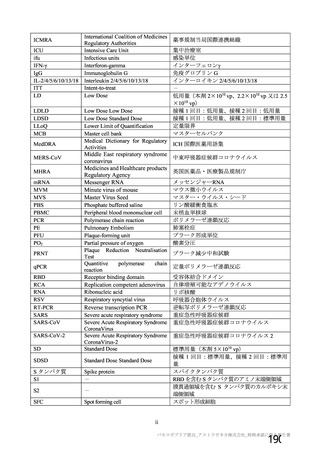
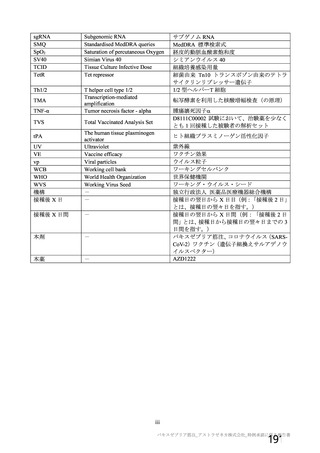
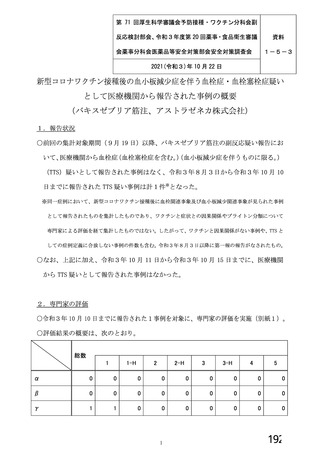
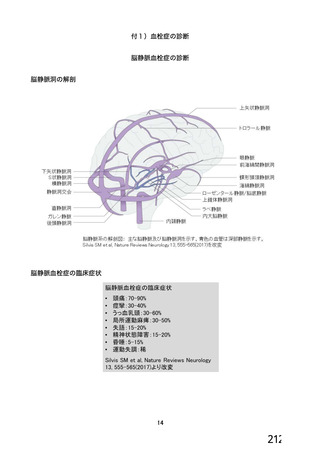
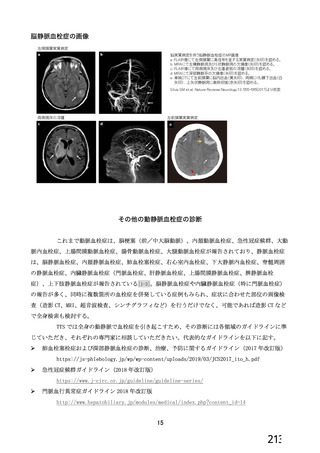
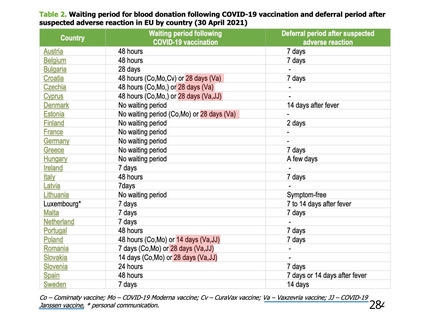
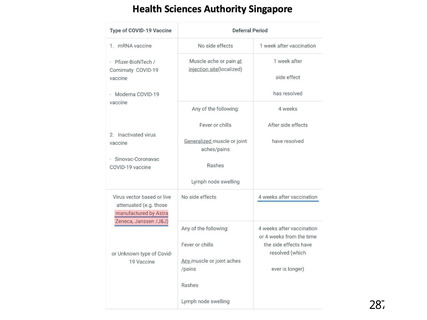
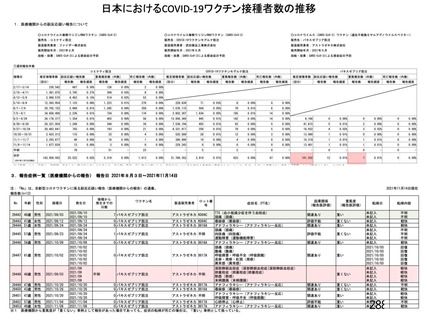
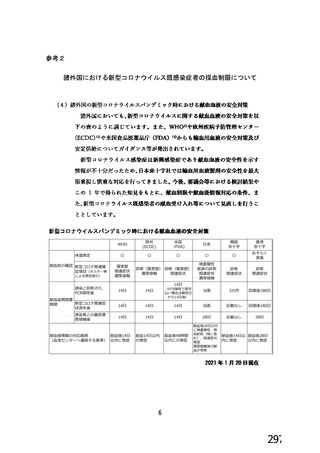
資 料4-1 令和3年度第6回安全技術調査会の審議結果について (321 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_26025.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会血液事業部会(令和4年度第1回 6/8)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
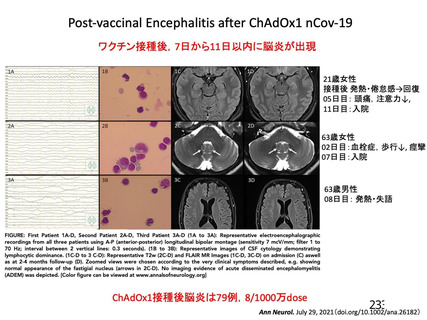
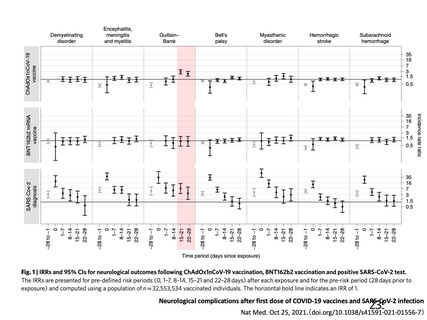
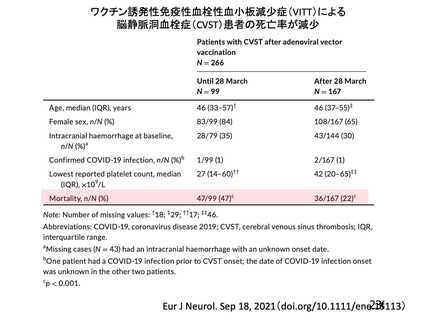
Thrombocytopenia Syndrome (TTS) following Adenovirus Vectored COVID-19 Vaccinations. 11 August
2021. https://www.un.org/sites/un2.un.org/files/coronavirus_vipitguidance.pdf(2021/10/30 アクセ
ス)
12. Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med
2021;384:2254-2256. doi:10.1056/NEJMe2106315
13. https://www.nature.com/articles/d41586-021-00932-0(2021/10/30 アクセス)
14. Signal assessment report on embolic and thrombotic events (SMQ) with COVID-19 Vaccine (ChAdOx1S [recombinant]) –COVID-19 Vaccine AstraZeneca (Other viral vaccines). EPITT no:19683
https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-reportembolic-thrombotic-events-smq-covid-19-vaccine-chadox1-s-recombinant-covid_en.pdf(2021/10/30
アクセス)
15. Vaxzevria (COVID-19 Vaccine (ChAdOx1-S [recombinant])). An overview of Vaxzevria and why it is
authorised in the EU. https://www.ema.europa.eu/en/documents/overview/vaxzevria-previouslycovid-19-vaccine-astrazeneca-epar-medicine-overview_en.pdf(2021/10/30 アクセス)
16. Greinacher A. CLINICAL PRACTICE. Heparin-Induced Thrombocytopenia. N Engl J Med. 2015; 373: 25261. doi: 10.1056/NEJMcp1411910.
17. Greinacher A, Farner B, Kroll H, Kohlmann T, Warkentin TE, Eichler P. Clinical features of heparininduced thrombocytopenia including risk factors for thrombosis. A retrospective analysis of 408
patients. Thromb Haemost. 2005; 94: 132-135. doi: 10.1160/TH04-12-0825.
18. Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb
Haemost. 2017; 15: 2099-2114. doi: 10.1111/jth.13813.
19. Sørensen, ALT, Rolland M, Hartmann J, et al.: A case of thrombocytopenia and multiple thromboses after
vaccination with ChAdOx1 nCoV-19 against SARS-CoV-2. Blood Adv 5: 2569-2574, 2021. doi:
10.1182/bloodadvances.2021004904.
20. Blauenfeldt RA, Kristensen SR, Ernstsen SL, et al.: Thrombocytopenia with acute ischemic stroke and
bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb
Haemost 19: 1771-1775, 2021. doi: 10.1111/jth.15347.
21. Tiede A, Sachs UJ, Czwalinna A, Werwitzke S, Bikker R, Krauss JK, Donnerstag FG, Weißenborn K,
Höglinger GU, Maasoumy B, Wedemeyer H, Ganser A. Prothrombotic immune thrombocytopenia after
COVID-19 vaccine. Blood. 2021;138:350-353. doi: 10.1182/blood.2021011958.
22. Platton S, Bartlett A, MacCallum P, Makris M, McDonald V, Singh D, Scully M, Pavord S. Evaluation of
laboratory assays for anti-Platelet Factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J Thromb
Haemost. 2021;19:2007-2013. doi: 10.1111/jth.15362.
23. Thiele T, Ulm L, Holtfreter S, Schönborn L, Kuhn SO, Scheer C, Warkentin TE, Bröker B, Becker K, Aurich
K, Selleng K, Hübner NO, Greinacher A. Frequency of positive anti-PF4/polyanion antibody tests after
20
319