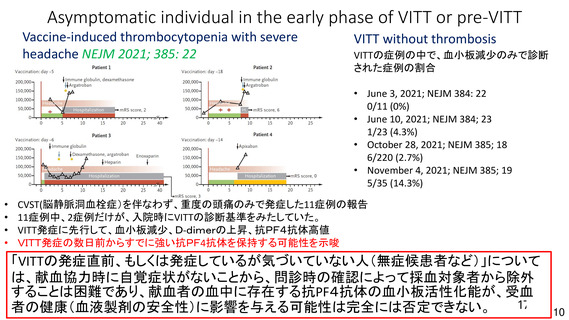
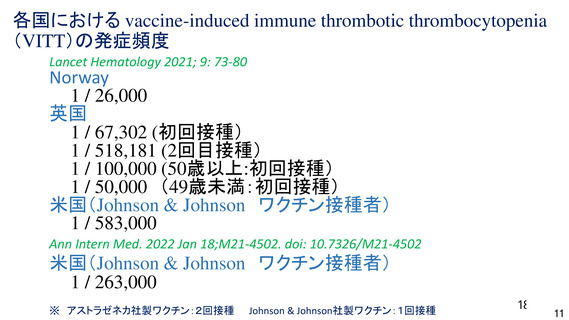
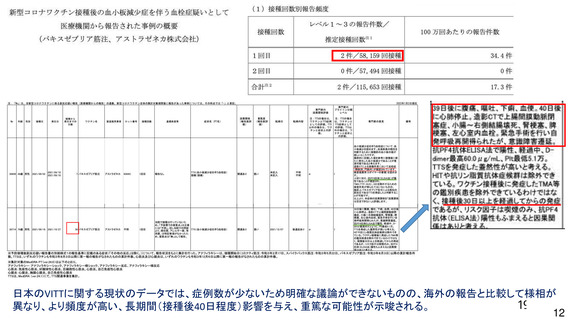
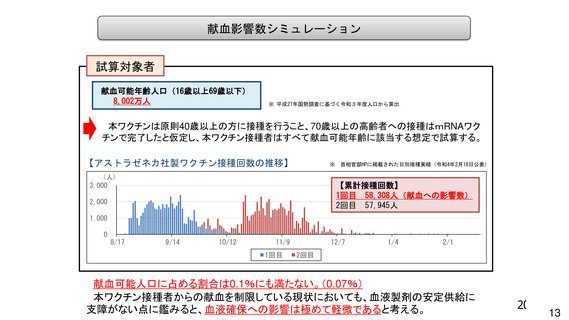
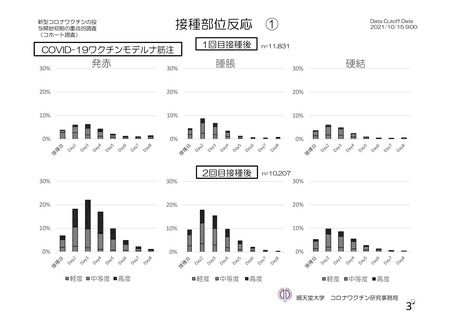
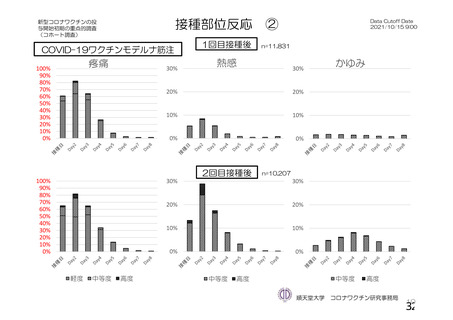
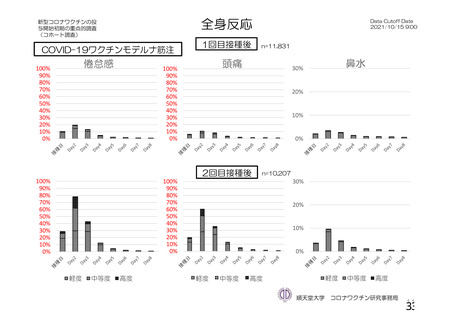
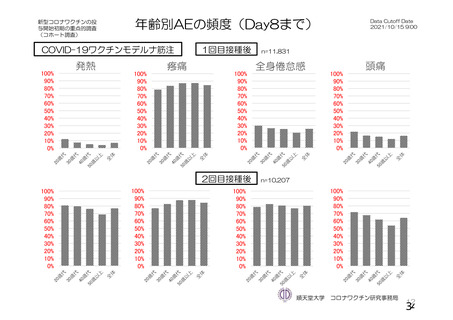
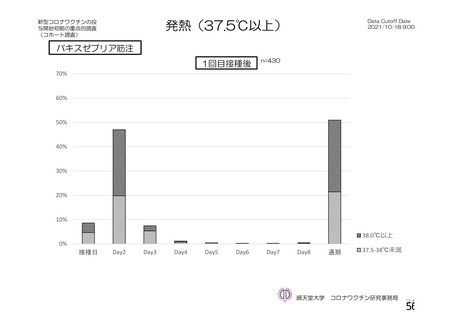
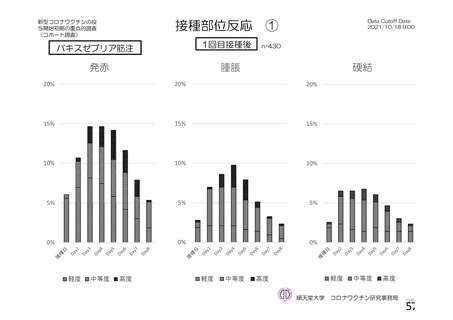
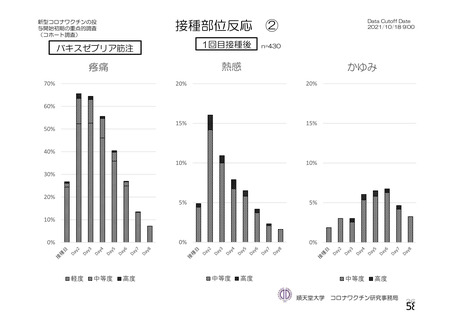
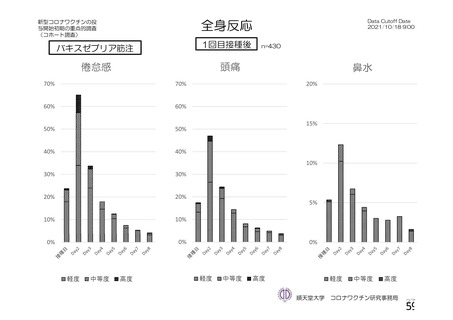
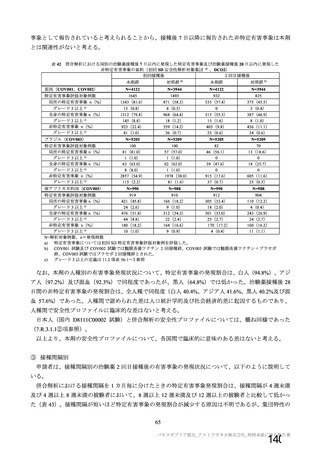
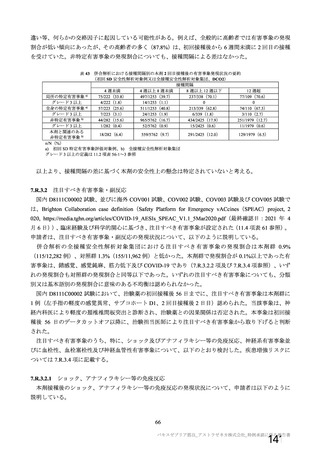
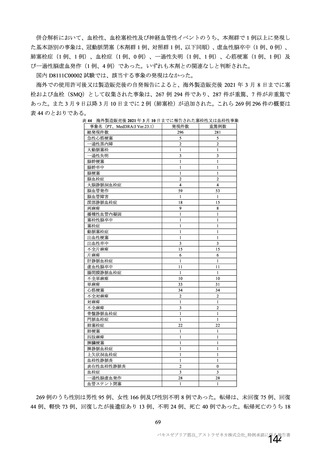
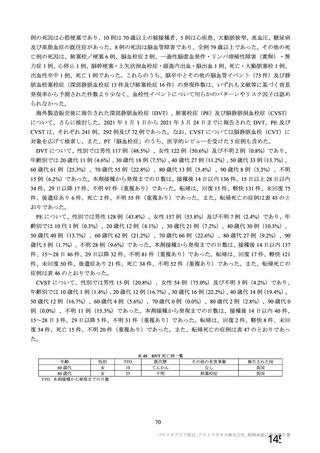
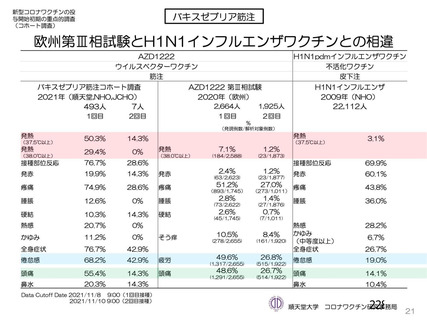
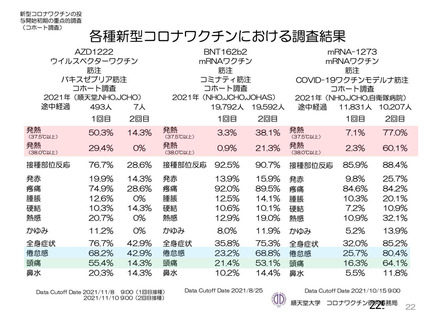
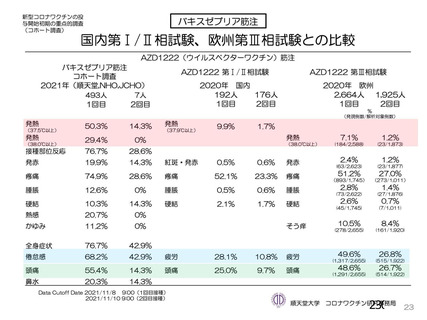
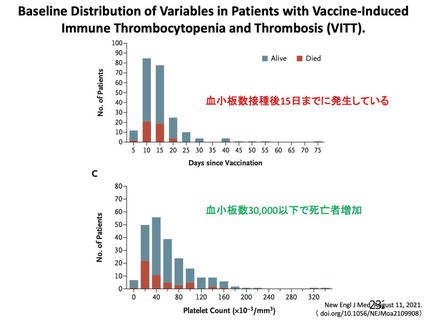
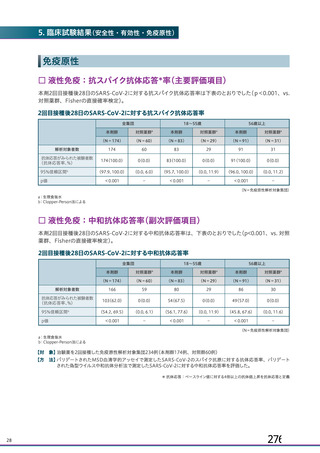
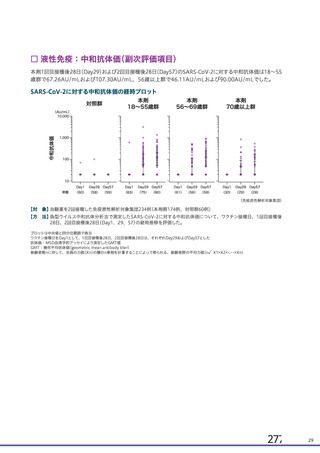
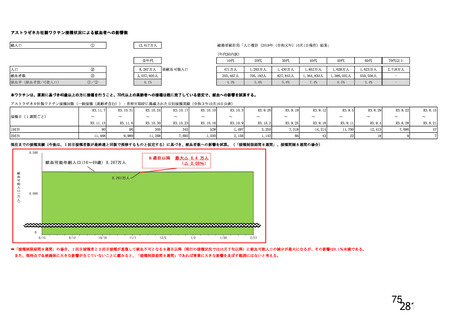
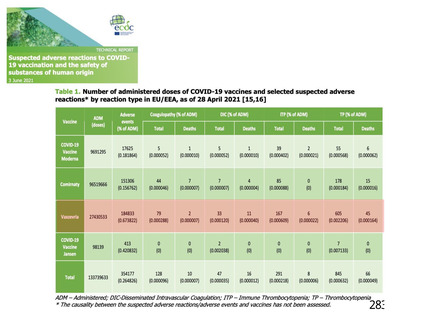
資 料4-1 令和3年度第6回安全技術調査会の審議結果について (220 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_26025.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会血液事業部会(令和4年度第1回 6/8)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
heparin-induced thrombocytopenia: a propensity score-matched study. Blood. 2015; 125:924-929.
25. Warkentin TE, Sheppard JA, Chu FV, Kapoor A, Crowther MA, Gangji A. Plasma exchange to remove HIT
antibodies: dissociation between enzyme-immunoassay and platelet activation test reactivities. Blood.
2015; 125: 195-198. doi: 10.1182/blood-2014-07-590844.
26. Ferro JM, Coutinho JM, Dentali F, Kobayashi A, Alasheev A, Canhão P, Karpov D, Nagel S, Posthuma L,
Roriz JM, Caria J, Frässdorf M, Huisman H, Reilly P, Diener HC ; for the RE-SPECT CVT Study Group. Safety
and Efficacy of Dabigatran Etexilate vs Dose-Adjusted Warfarin in Patients With Cerebral Venous
Thrombosis: A Randomized Clinical Trial. JAMA Neurol. 2019; 76: 1457-1465.
27. Lee GKH, Chen VH, Tan CH, Leow AST, Kong WY, Sia CH, Chew NWS, Tu TM, Chan BPL, Yeo LLL, Sharma
VK, TanBYQ. Comparing the efficacy and safety of direct oral anticoagulants with vitamin k antagonist
in cerebral venous thrombosis. J Thromb Thrombolysis. 2020; 50: 724-731. doi: 10.1007/s11239-02002106-7.
28. American Heart Association/American Stroke Association Stroke Council Leadership. Diagnosis and
Management
of
Cerebral
Venous
Sinus
Thrombosis
with
Vaccine-Induced
Thrombotic
Thrombocytopenia. Stroke. 2021 Apr 29. doi: 10.1161/STROKEAHA.121.035564.
29. Caprio F, Bernstein RA. Duration of Anticoagulation After Cerebral Venous Sinus Thrombosis. Neurocrit
Care. 2012; 16: 335-342. doi: 10.1007/s12028-011-9661-1.
30. Pavord S, Lester W, Makris M, Scully M, Hunt B. Guidance from the Expert Haematology Panel (EHP) on
Covid-19 Vaccine-induced Immune Thrombocytopenia and Thrombosis (VITT). (Updated Guidance on
Management. Version 1.7, 20 April, 2021). https://b-s-h.org.uk/media/19590/guidance-version-17on-mngmt-of-vitt-20210420.pdf(2021/05/26 アクセス)
31. Viegas LD, Stolz E, Canhão P, Ferro JM. Systemic thrombolysis for cerebral venous and dural sinus
thrombosis: a systematic review. Cerebrovasc Dis. 2014; 37: 43-50. doi: 10.1159/000356840.
32. Stam J, Majoie CBLM, van Delden OM, van Lienden KP, Reekers JA. Endovascular thrombectomy and
thrombolysis for severe cerebral sinus thrombosis: a prospective study. Stroke. 2008; 39: 1487-1490.
doi: 10.1161/STROKEAHA.107.502658.
33. Dentali F, Squizzato A, Gianni M, De Lodovici ML, Venco A, Paciaroni M, Crowther M, Ageno W. Safety of
thrombolysis in cerebral venous thrombosis. A systematic review of the literature. Thromb Haemost.
2010; 104: 1055-1062. doi: 10.1160/TH10-05-0311.
34. 日本脳卒中学会 脳卒中ガイドライン委員会: 脳卒中治療ガイドライン 2015[追補 2019 対応]協
和企画
東京
35. Siddiqui FM, Dandapat S, Banerjee C, Zuurbier SM, Johnson M, Stam J, Coutinho JM. Mechanical
thrombectomy in cerebral venous thrombosis: systematic review of 185 cases. Stroke. 2015; 46: 12631268.
20
218