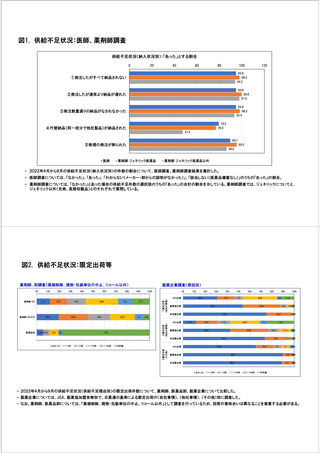
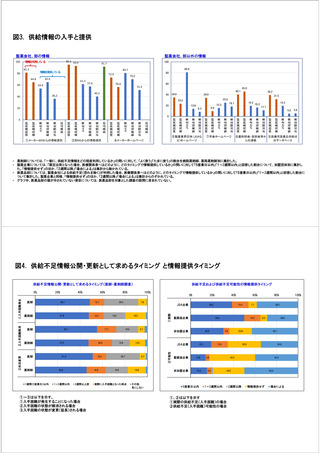
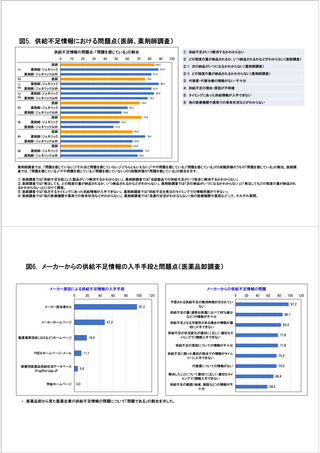
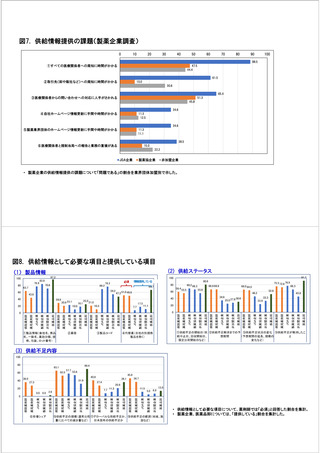
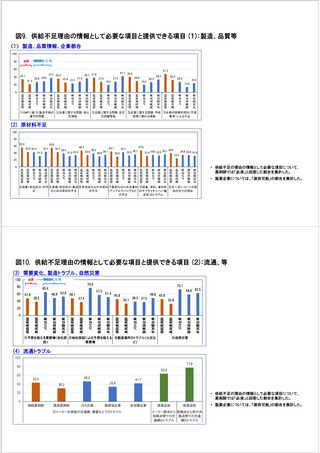
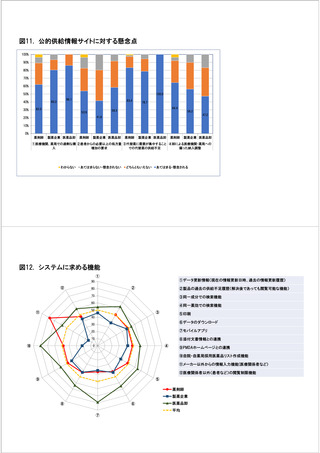
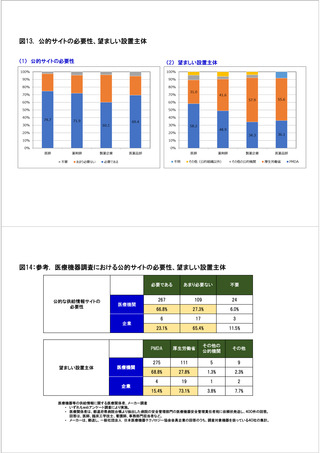
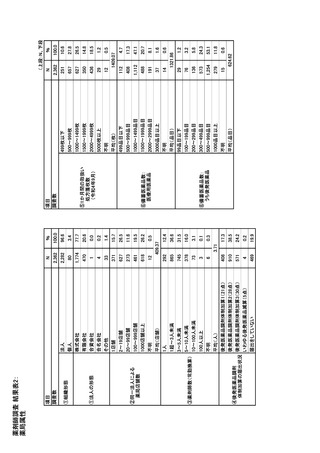
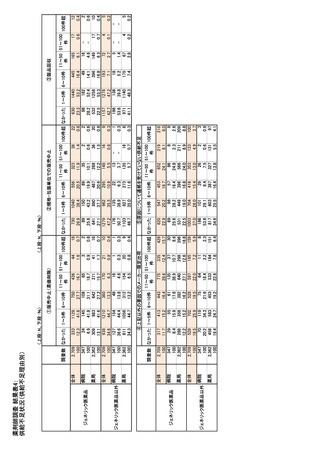
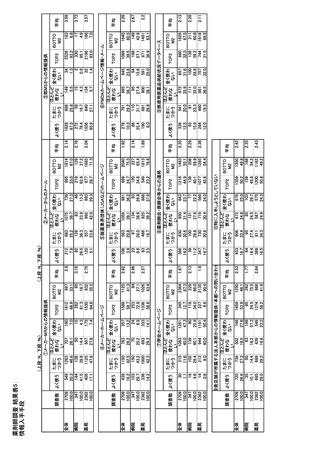
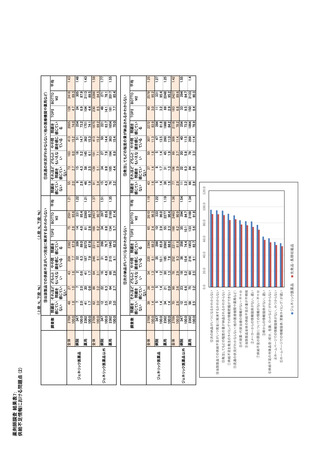
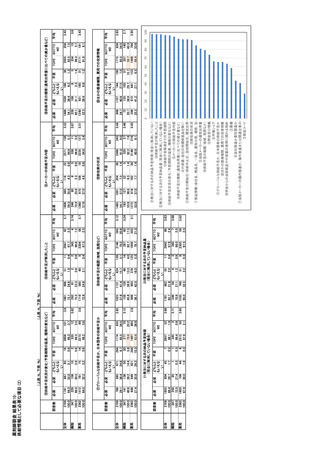
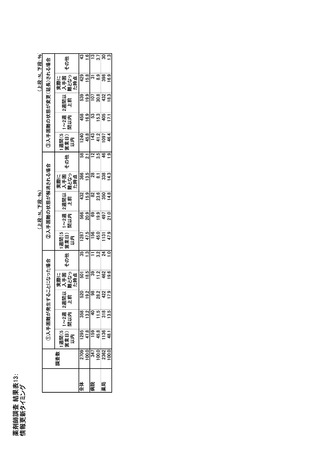
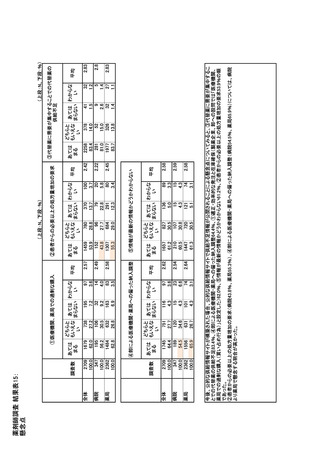
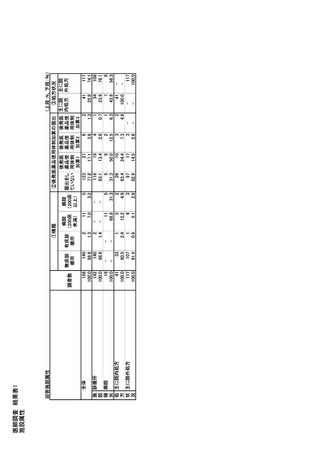
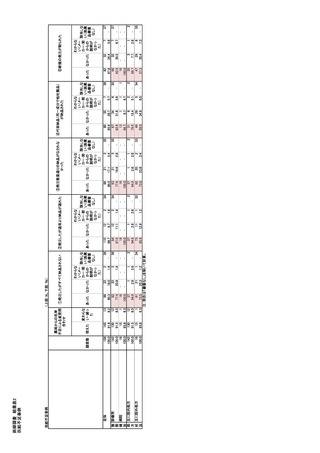
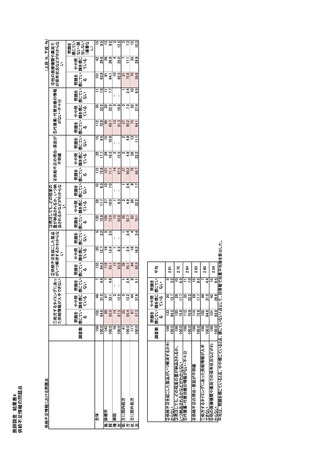
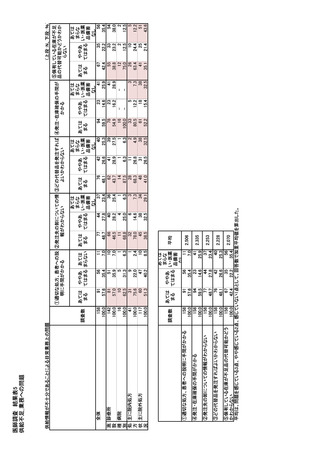
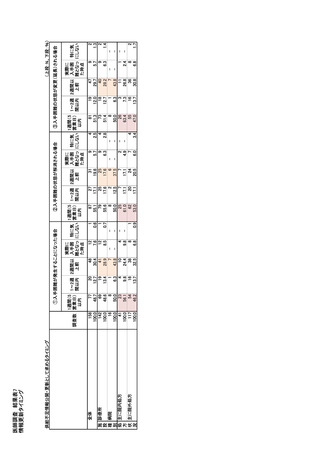
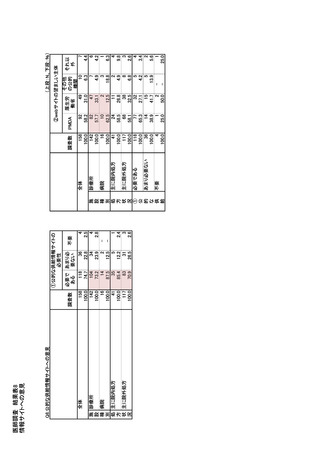
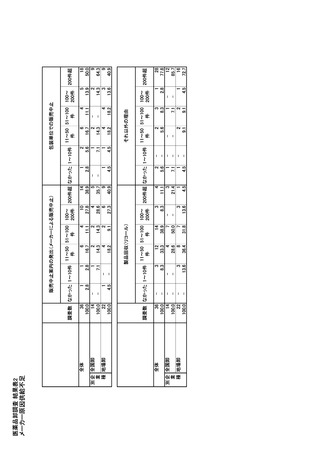
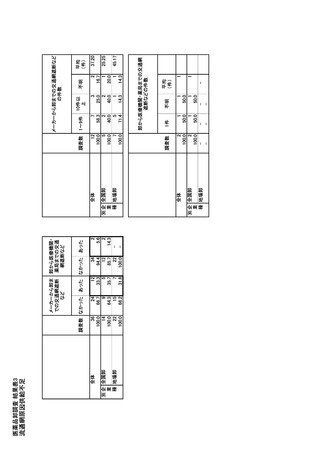
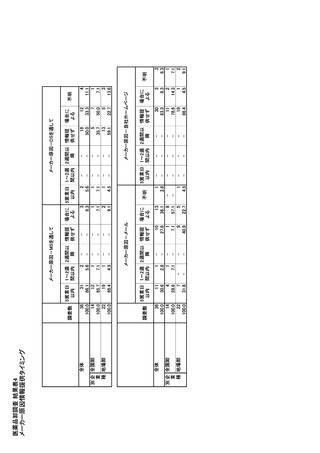
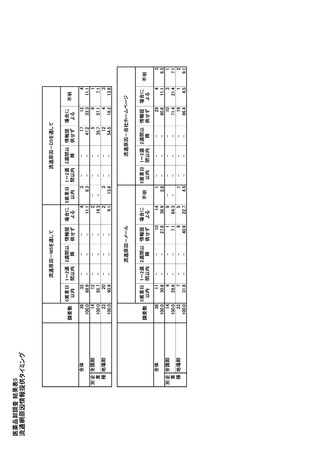
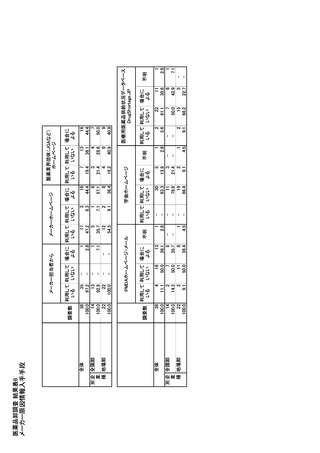
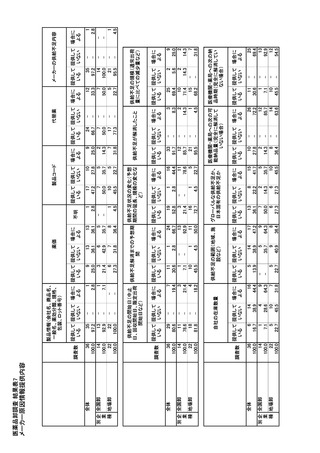
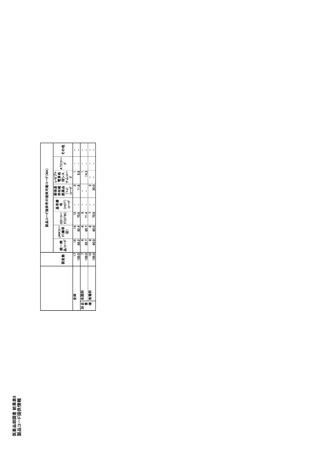
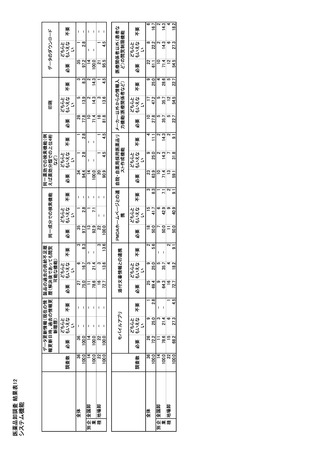
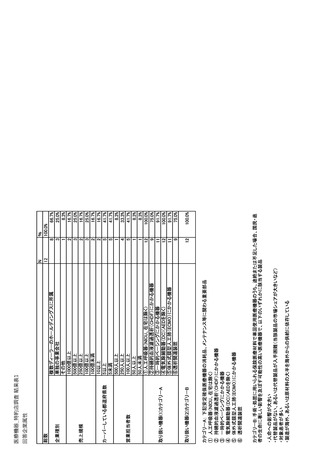
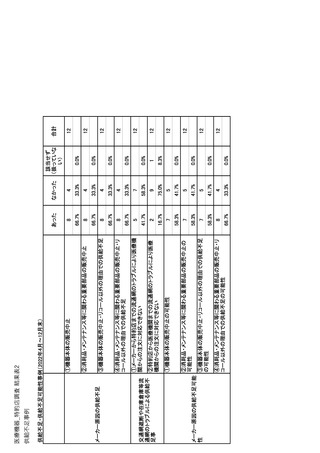
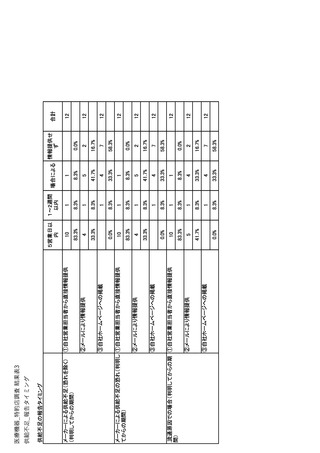
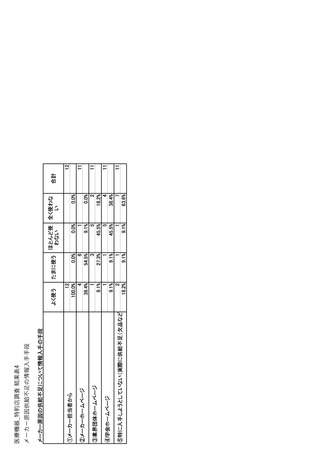
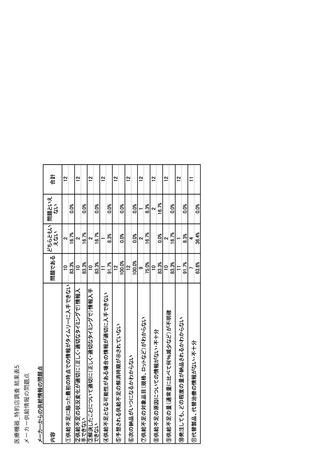
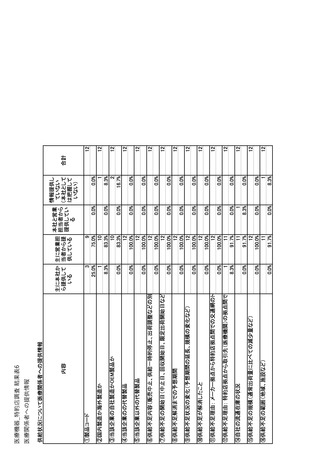
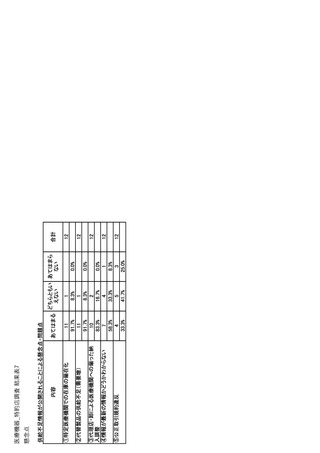
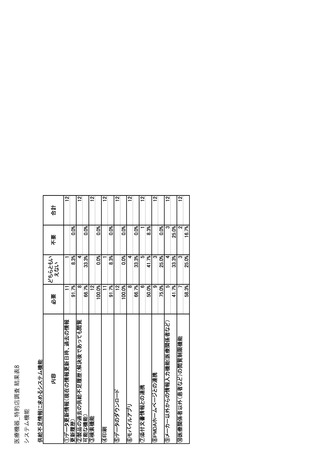
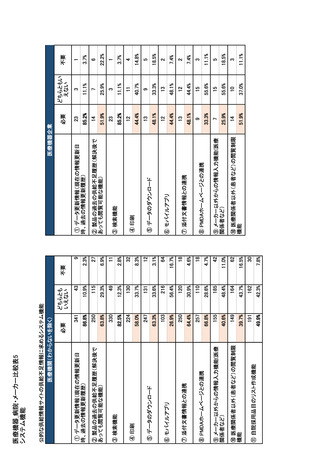
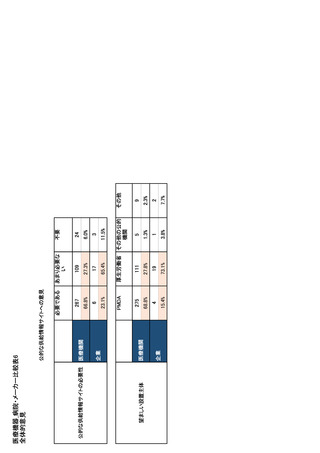
参考資料6 令和4年度厚生労働科学特別研究事業「医療用医薬品・医療機器等の供給情報を医療従 事者等へ適切に提供するための情報システムの構築に向けた研究」(研究代表者:坂巻弘之) (247 ページ)
出典
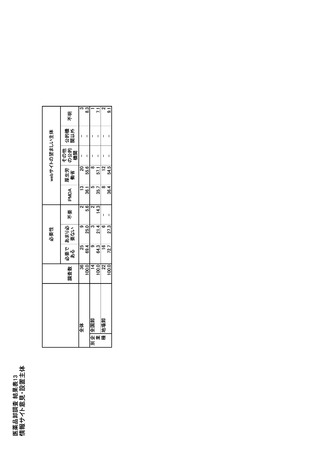
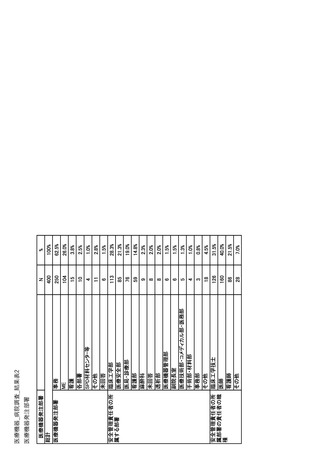
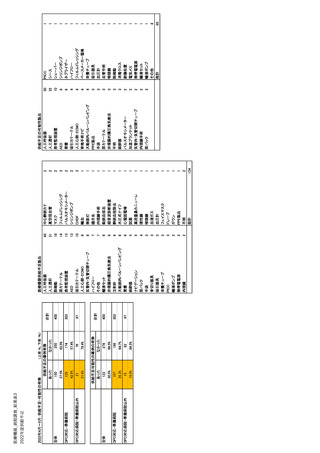
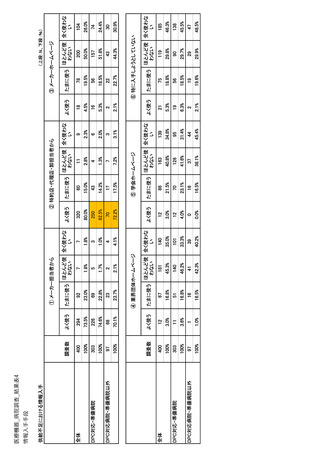
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35103.html |
| 出典情報 | 医療用医薬品の安定確保策に関する関係者会議 供給情報ワーキンググループ(第1回 9/7)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
https://health.ec.europa.eu/system/files/202210/mp_vulnerabilities_global-supply_swd_en.pdf
14) https://www.ema.europa.eu/en/documents/regulatoryprocedural-guideline/good-practices-industryprevention-human-medicinal-product-shortages_en.pdf
15) Guidance for industry to prevent and mitigate medicine
shortages
https://www.ema.europa.eu/en/news/guidance-industryprevent-mitigate-medicine-shortages
16) Good practice guidance for patient and healthcare
professional organisations on the prevention of shortages
of medicines for human use
https://www.ema.europa.eu/en/documents/other/goodpractice-guidance-patient-healthcare-professionalorganisations-prevention-shortages-medicines_en.pdf
17) Executive Steering Group on Shortages of Medical
Devices
https://www.ema.europa.eu/en/about-us/what-wedo/crisis-preparedness-management/executive-steeringgroup-shortages-safety-medical-devices-mdssg
1) HMA / EMA Task Force on the Availability of Authorised
Medicines for Human and Veterinary Use:
https://www.hma.eu/fileadmin/dateien/HMA_joint/00_About_HMA/03Working_Groups/TF_Availability/2022_09_HMAEMA_TF_Availability_ToR.pdf
2) European medicines agencies network strategy:
https://www.ema.europa.eu/en/about-us/how-wework/european-medicines-regulatory-network/europeanmedicines-agencies-network-strategy#network-strategyto-2025-section
3) European medicines agencies network strategy to 2025
https://www.ema.europa.eu/en/documents/report/europ
ean-union-medicines-agencies-network-strategy-2025protecting-public-health-time-rapid-change_en.pdf
4) Pharmaceutical Strategy for Europe:
https://health.ec.europa.eu/medicinalproducts/pharmaceutical-strategy-europe_en
5) European Commission: Future-proofing pharmaceutical
legislation —study on medicine shortages Final report。
https://op.europa.eu/en/publication-detail//publication/e964d173-5320-11ec-91ac-01aa75ed71a1
6) HMA/EMA (2019) Good practice guidance for
communication to the public on medicines’ availability
issues.
https://www.ema.europa.eu/en/documents/regulatoryprocedural-guideline/good-practice-guidancecommunication-public-medicines-availabilityissues_en.pdf
7) Executive Steering Group on Shortages and Safety of
Medicinal Products:
https://www.ema.europa.eu/en/about-us/what-wedo/crisis-preparedness-management/executive-steeringgroup-shortages-safety-medicinal-products
8) Medicine Shortages Single Point of Contact (SPOC)
Working Party:Medicine Shortages Single Point of
Contact (SPOC) Working Party
9) Regulation (EU) 2022/123 of the European Parliament and
of the Council of 25 January 2022 on a reinforced role for
the European Medicines Agency in crisis preparedness
and management for medicinal products and medical
devices https://eur-lex.europa.eu/legalcontent/EN/TXT/?uri=CELEX%3A32022R0123
10) List of the “main therapeutic groups” (MTGs) in crisis
preparedness:
https://www.ema.europa.eu/en/documents/other/listmain-therapeutic-groups-mtgs-crisis-preparedness_en.pdf
11) Public information on medicine shortages:
https://www.ema.europa.eu/en/human-regulatory/postauthorisation/availability-medicines/public-informationmedicine-shortages#ema-shortages-catalogue-section
12) European Health Union: Commission proposes
pharmaceuticals reform for more accessible, affordable and
innovative medicines:
https://ec.europa.eu/commission/presscorner/detail/en/
IP_23_1843
13) COMMISSION STAFF WORKING DOCUMENT:
245