よむ、つかう、まなぶ。
資料5-3 Ⅳ-144、145 フルダラビン[1.7MB] (95 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00044.html |
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第66回 12/12)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
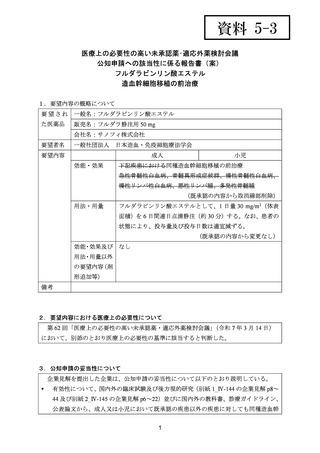
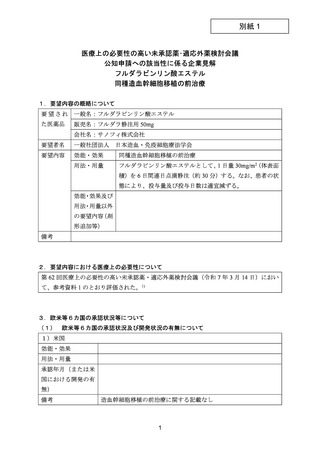
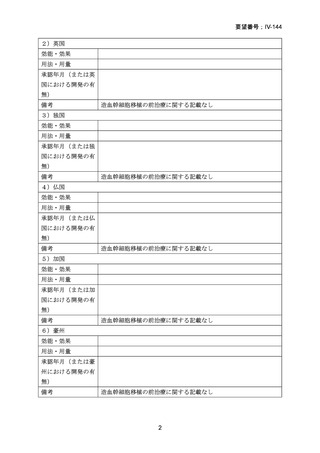
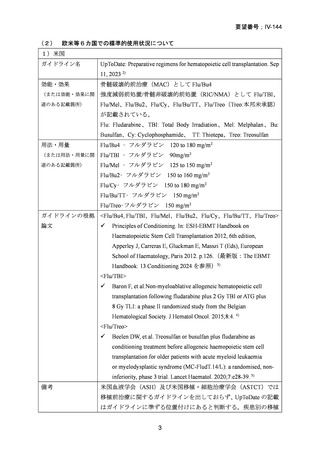
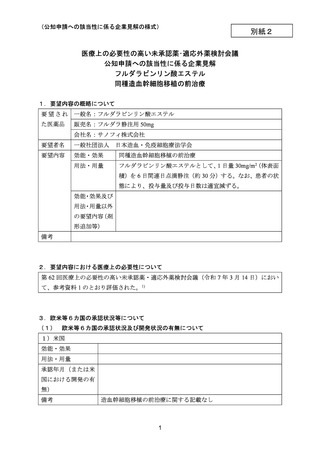
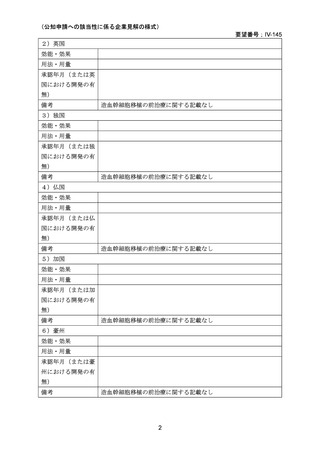
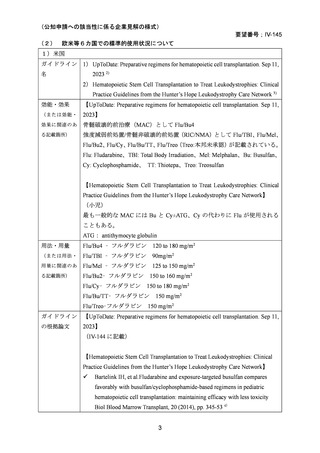
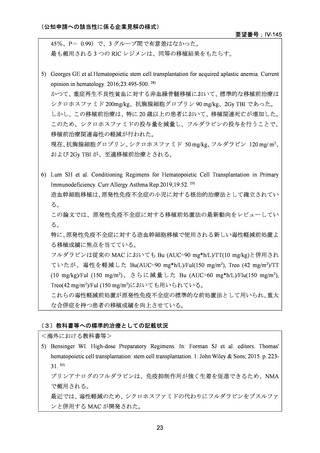
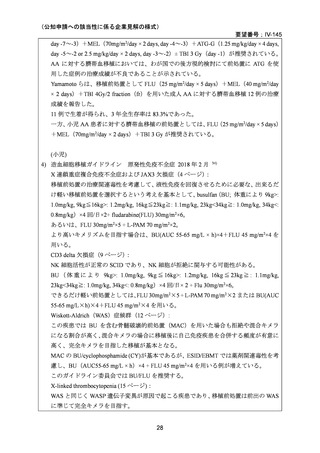
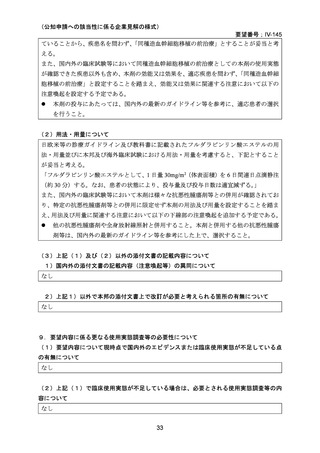
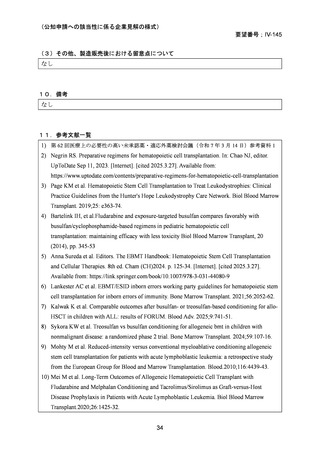
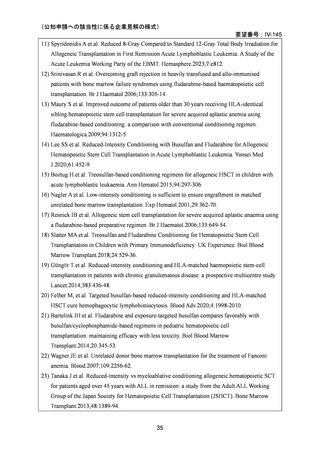
(公知申請への該当性に係る企業見解の様式)
要望番号;IV-145
(3)その他、製造販売後における留意点について
なし
10.備考
なし
11.参考文献一覧
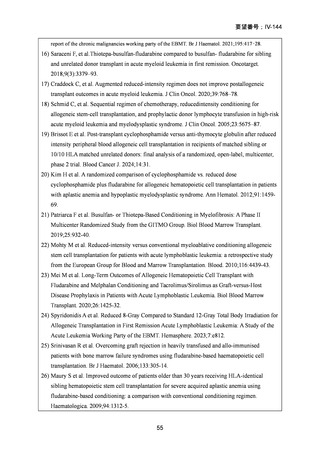
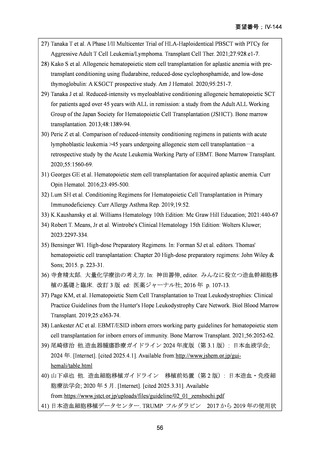
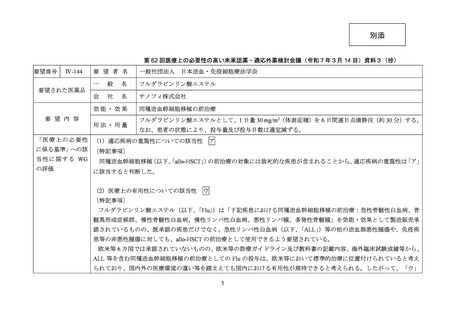
1) 第 62 回医療上の必要性の高い未承認薬・適応外薬検討会議(令和 7 年 3 月 14 日)参考資料 1
2) Negrin RS. Preparative regimens for hematopoietic cell transplantation. In: Chao NJ, editor.
UpToDate Sep 11, 2023. [Internet]. [cited 2025.3.27]. Available from:
https://www.uptodate.com/contents/preparative-regimens-for-hematopoietic-cell-transplantation
3) Page KM et al. Hematopoietic Stem Cell Transplantation to Treat Leukodystrophies: Clinical
Practice Guidelines from the Hunter's Hope Leukodystrophy Care Network. Biol Blood Marrow
Transplant. 2019;25: e363-74.
4) Bartelink IH, et al.Fludarabine and exposure-targeted busulfan compares favorably with
busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell
transplantation: maintaining efficacy with less toxicity Biol Blood Marrow Transplant, 20
(2014), pp. 345-53
5) Anna Sureda et al. Editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation
and Cellular Therapies. 8th ed. Cham (CH)2024. p. 125-34. [Internet]. [cited 2025.3.27].
Available from: https://link.springer.com/book/10.1007/978-3-031-44080-9
6) Lankester AC et al. EBMT/ESID inborn errors working party guidelines for hematopoietic stem
cell transplantation for inborn errors of immunity. Bone Marrow Transplant. 2021;56:2052-62.
7) Kalwak K et al. Comparable outcomes after busulfan- or treosulfan-based conditioning for alloHSCT in children with ALL: results of FORUM. Blood Adv. 2025;9:741-51.
8) Sykora KW et al. Treosulfan vs busulfan conditioning for allogeneic bmt in children with
nonmalignant disease: a randomized phase 2 trial. Bone Marrow Transplant. 2024;59:107-16.
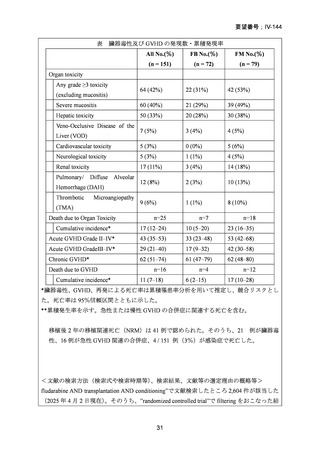
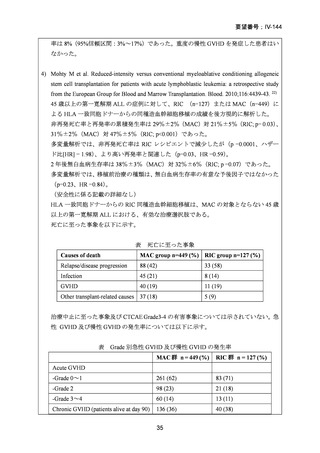
9) Mohty M et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic
stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study
from the European Group for Blood and Marrow Transplantation. Blood.2010;116:4439-43.
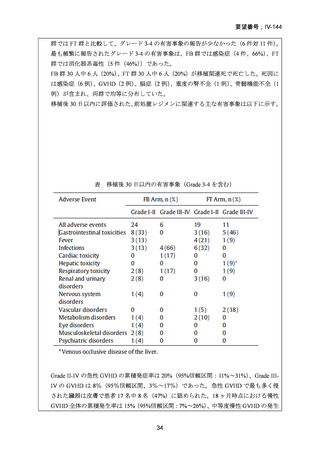
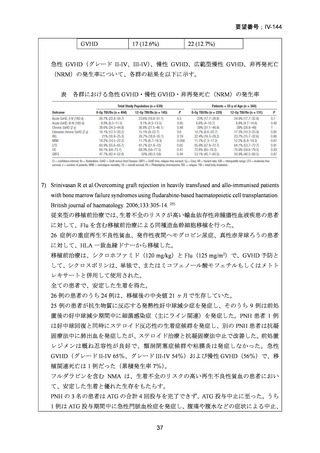
10) Mei M et al. Long-Term Outcomes of Allogeneic Hematopoietic Cell Transplant with
Fludarabine and Melphalan Conditioning and Tacrolimus/Sirolimus as Graft-versus-Host
Disease Prophylaxis in Patients with Acute Lymphoblastic Leukemia. Biol Blood Marrow
Transplant.2020;26:1425-32.
34
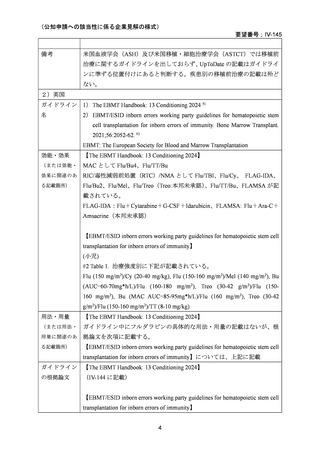
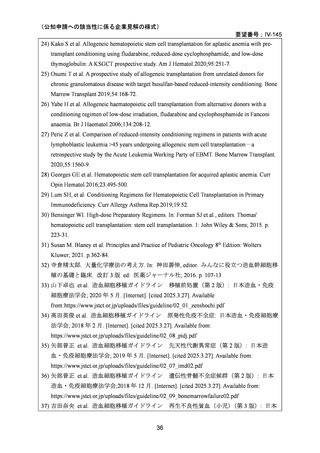
要望番号;IV-145
(3)その他、製造販売後における留意点について
なし
10.備考
なし
11.参考文献一覧
1) 第 62 回医療上の必要性の高い未承認薬・適応外薬検討会議(令和 7 年 3 月 14 日)参考資料 1
2) Negrin RS. Preparative regimens for hematopoietic cell transplantation. In: Chao NJ, editor.
UpToDate Sep 11, 2023. [Internet]. [cited 2025.3.27]. Available from:
https://www.uptodate.com/contents/preparative-regimens-for-hematopoietic-cell-transplantation
3) Page KM et al. Hematopoietic Stem Cell Transplantation to Treat Leukodystrophies: Clinical
Practice Guidelines from the Hunter's Hope Leukodystrophy Care Network. Biol Blood Marrow
Transplant. 2019;25: e363-74.
4) Bartelink IH, et al.Fludarabine and exposure-targeted busulfan compares favorably with
busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell
transplantation: maintaining efficacy with less toxicity Biol Blood Marrow Transplant, 20
(2014), pp. 345-53
5) Anna Sureda et al. Editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation
and Cellular Therapies. 8th ed. Cham (CH)2024. p. 125-34. [Internet]. [cited 2025.3.27].
Available from: https://link.springer.com/book/10.1007/978-3-031-44080-9
6) Lankester AC et al. EBMT/ESID inborn errors working party guidelines for hematopoietic stem
cell transplantation for inborn errors of immunity. Bone Marrow Transplant. 2021;56:2052-62.
7) Kalwak K et al. Comparable outcomes after busulfan- or treosulfan-based conditioning for alloHSCT in children with ALL: results of FORUM. Blood Adv. 2025;9:741-51.
8) Sykora KW et al. Treosulfan vs busulfan conditioning for allogeneic bmt in children with
nonmalignant disease: a randomized phase 2 trial. Bone Marrow Transplant. 2024;59:107-16.
9) Mohty M et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic
stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study
from the European Group for Blood and Marrow Transplantation. Blood.2010;116:4439-43.
10) Mei M et al. Long-Term Outcomes of Allogeneic Hematopoietic Cell Transplant with
Fludarabine and Melphalan Conditioning and Tacrolimus/Sirolimus as Graft-versus-Host
Disease Prophylaxis in Patients with Acute Lymphoblastic Leukemia. Biol Blood Marrow
Transplant.2020;26:1425-32.
34