よむ、つかう、まなぶ。
資料5-3 Ⅳ-144、145 フルダラビン[1.7MB] (58 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00044.html |
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第66回 12/12)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
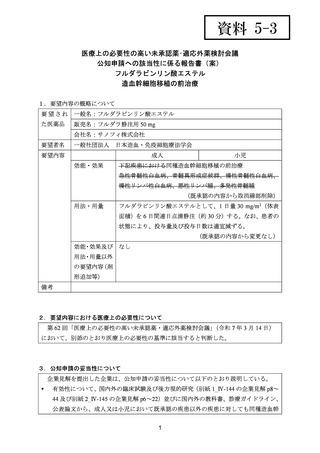
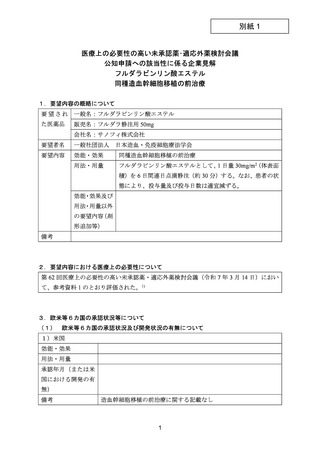
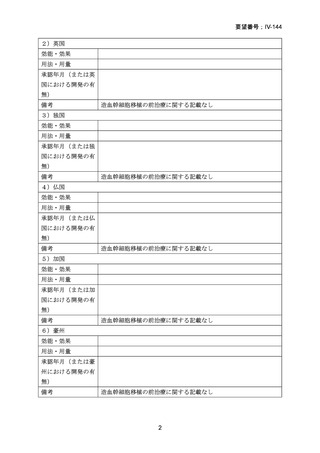
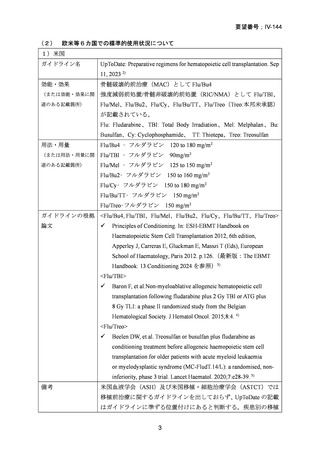
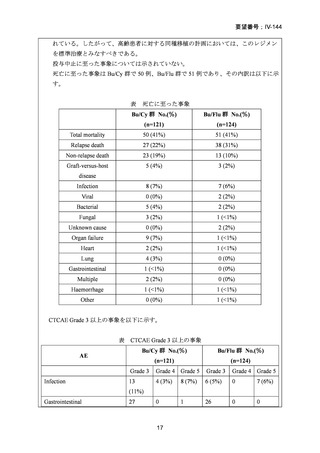
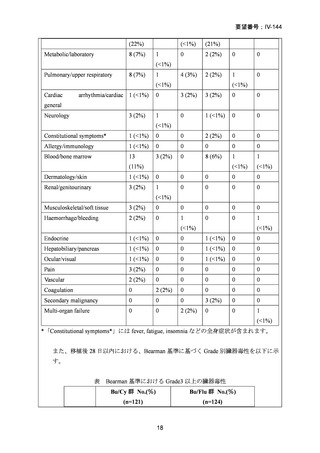
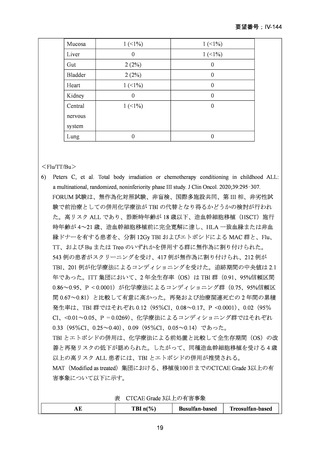
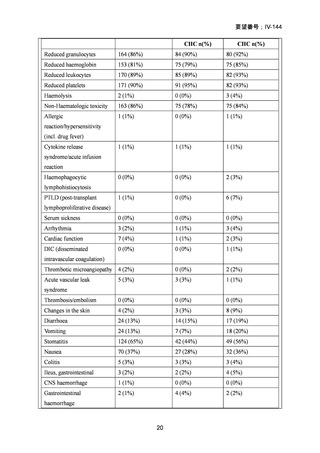
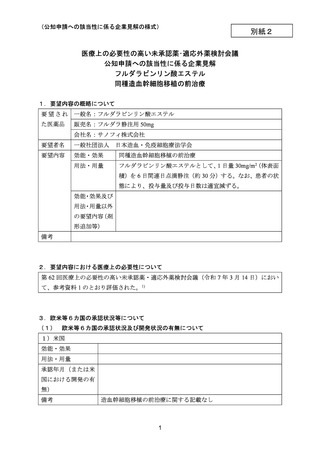
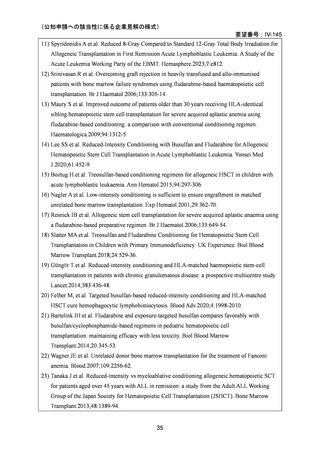
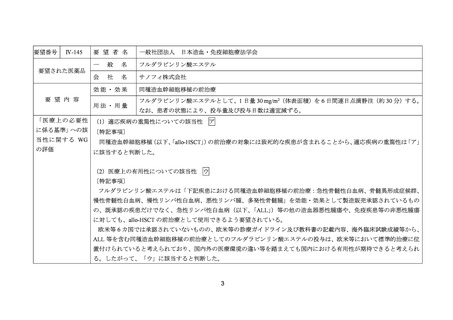
要望番号;IV-144
and Cellular Therapies. 2nd ed. Cham (CH)2024. p. 125-134. [Internet]. [cited 2025.3.31].
Available from: https://link.springer.com/book/10.1007/978-3-031-44080-9
4) Baron F, et al. Non-myeloablative allogeneic hematopoietic cell transplantation following
fludarabine plus 2 Gy TBI or ATG plus 8 Gy TLI: a phase II randomized study from the Belgian
Hematological Society. J Hematol Oncol. 2015;8:4.
5) Beelen DW, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic
haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia
or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet
Haematol. 2020;7:e28–39.
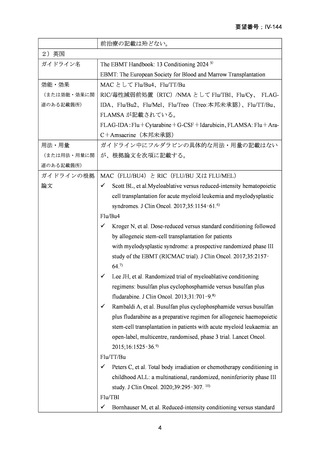
6) Scott BL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for
acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–61.
7) Kroger N, et al. Dose-reduced versus standard conditioning followed by allogeneic stem-cell
transplantation for patients with myelodysplastic syndrome: a prospective randomized phase III
study of the EBMT (RICMAC trial). J Clin Oncol. 2017;35:2157–64.
8) Lee JH, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus
cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013;31:701–9.
9) Rambaldi A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a
preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with
acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol.
2015;16:1525–36.
10) Peters C, et al. Total body irradiation or chemotherapy conditioning in childhood ALL:
a multinational, randomized, noninferiority phase III study. J Clin Oncol. 2020;39:295–307.
11) Bornhauser M, et al. Reduced-intensity conditioning versus standard conditioning before
allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first
complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol.
2012;13:1035–44.
12) Blaise D, et al. Randomized study of 2 reduced-intensity conditioning strategies for human
leukocyte antigen-matched, related allogeneic peripheral blood stem cell transplantation:
prospective clinical and socioeconomic evaluation. Cancer. 2013;119:602–11.
13) Eapen M, et al. Hematopoietic cell transplant for acute myeloid leukemia and myelodysplastic
syndrome: conditioning regimen intensity. Blood Adv. 2018;2:2095–103.
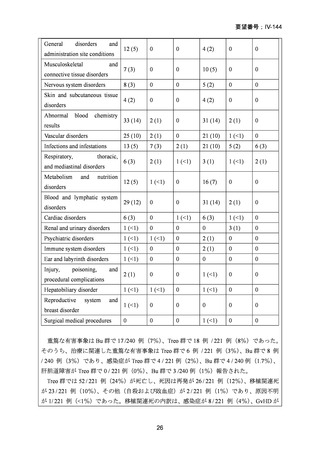
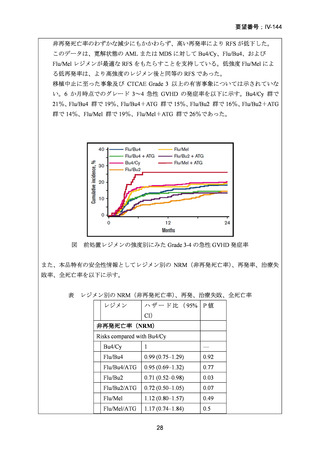
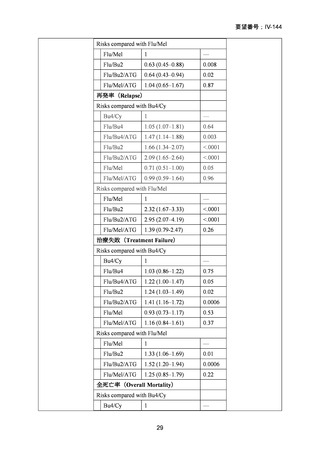
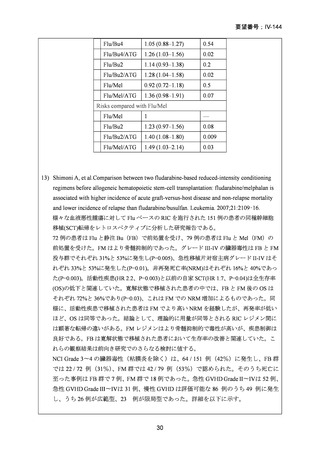
14) Shimoni A, et al. Comparison between two fludarabine-based reduced-intensity conditioning
regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is
associated with higher incidence of acute graft-versus-host disease and non-relapse mortality
and lower incidence of relapse than fludarabine/busulfan. Leukemia. 2007;21:2109–16.
15) Shimoni A, et al.Allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome
using treosulfan based compared to other reduced-intensity or myeloablative conditioning regimens. A
54
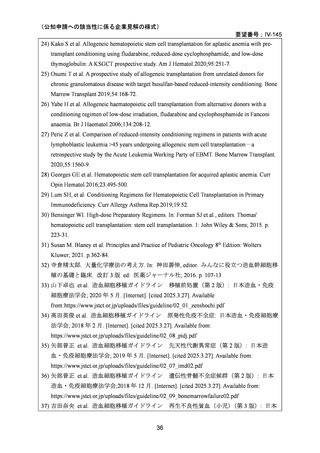
and Cellular Therapies. 2nd ed. Cham (CH)2024. p. 125-134. [Internet]. [cited 2025.3.31].
Available from: https://link.springer.com/book/10.1007/978-3-031-44080-9
4) Baron F, et al. Non-myeloablative allogeneic hematopoietic cell transplantation following
fludarabine plus 2 Gy TBI or ATG plus 8 Gy TLI: a phase II randomized study from the Belgian
Hematological Society. J Hematol Oncol. 2015;8:4.
5) Beelen DW, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic
haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia
or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet
Haematol. 2020;7:e28–39.
6) Scott BL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for
acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–61.
7) Kroger N, et al. Dose-reduced versus standard conditioning followed by allogeneic stem-cell
transplantation for patients with myelodysplastic syndrome: a prospective randomized phase III
study of the EBMT (RICMAC trial). J Clin Oncol. 2017;35:2157–64.
8) Lee JH, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus
cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013;31:701–9.
9) Rambaldi A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a
preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with
acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol.
2015;16:1525–36.
10) Peters C, et al. Total body irradiation or chemotherapy conditioning in childhood ALL:
a multinational, randomized, noninferiority phase III study. J Clin Oncol. 2020;39:295–307.
11) Bornhauser M, et al. Reduced-intensity conditioning versus standard conditioning before
allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first
complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol.
2012;13:1035–44.
12) Blaise D, et al. Randomized study of 2 reduced-intensity conditioning strategies for human
leukocyte antigen-matched, related allogeneic peripheral blood stem cell transplantation:
prospective clinical and socioeconomic evaluation. Cancer. 2013;119:602–11.
13) Eapen M, et al. Hematopoietic cell transplant for acute myeloid leukemia and myelodysplastic
syndrome: conditioning regimen intensity. Blood Adv. 2018;2:2095–103.
14) Shimoni A, et al. Comparison between two fludarabine-based reduced-intensity conditioning
regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is
associated with higher incidence of acute graft-versus-host disease and non-relapse mortality
and lower incidence of relapse than fludarabine/busulfan. Leukemia. 2007;21:2109–16.
15) Shimoni A, et al.Allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome
using treosulfan based compared to other reduced-intensity or myeloablative conditioning regimens. A
54