よむ、つかう、まなぶ。
08参考資料1 ヒトパピローマウイルス(HPV)ワクチンファクトシート追補版 (69 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_63875.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第31回 9/25)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
based human papillomavirus vaccination programme in Singapore: A modelling-based costeffectiveness analysis. Vaccine. 2023;41(12):1934-42.
78.
Palmer C, Tobe K, Negishi Y, You X, Chen YT, Abe M. Health impact and cost
effectiveness of implementing gender-neutral HPV vaccination in Japan. J Med Econ.
2023;26(1):1546-54.
79.
Ng SS, Hutubessy R, Chaiyakunapruk N. Systematic review of cost-effectiveness studies
of human papillomavirus (HPV) vaccination: 9-Valent vaccine, gender-neutral and multiple age
cohort vaccination. Vaccine. 2018;36(19):2529-44.
80.
Cody P, Tobe K, Abe M, Elbasha EH. Public health impact and cost effectiveness of
routine and catch-up vaccination of girls and women with a nine-valent HPV vaccine in Japan: a
model-based study. BMC Infect Dis. 2021;21(1):11.
81.
Kitano T. Risk-Benefit Analysis of the 9-Valent HPV Vaccination for Adolescent Boys
from an Individual Perspective. Jpn J Infect Dis. 2022;75(2):114-20.
82.
Datta S, Pink J, Medley GF, Petrou S, Staniszewska S, Underwood M, et al. Assessing
the cost-effectiveness of HPV vaccination strategies for adolescent girls and boys in the UK. BMC
Infect Dis. 2019;19(1):552.
83.
WHO.
International
Agency
for
Research
on
Cancer.
Available
from:
https://gco.iarc.fr/today/online-analysis-table
84.
Ito Y, Miyashiro I, Ito H, Hosono S, Chihara D, Nakata-Yamada K, et al. Long-term
survival and conditional survival of cancer patients in Japan using population- based cancer
registry data. Cancer Sci. 2014;105(11):1480-6.
85.
Japan Society for Head and Neck Cancer Cancer Registry Committee. Report of Head
and Neck Cancer Registry of Japan Clinical Statistics of Registered Patients, 2020. Available from:
https://square.umin.ac.jp/~jshnc/pdf/HNCreport_2020.pdf
86.
Drolet M, Benard E, Perez N, Brisson M, Group HPVVIS. Population-level impact and
herd effects following the introduction of human papillomavirus vaccination programmes: updated
systematic review and meta-analysis. Lancet. 2019;394(10197):497-509.
87.
WHO.
HPV
Dashboard
2024.
Available
from:
https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/humanpapillomavirus-vaccines-(HPV)/hpv-clearing-house/hpv-dashboard.
88.
Colzani E, Johansen K, Johnson H, Pastore Celentano L. Human papillomavirus
vaccination in the European Union/European Economic Area and globally: a moral dilemma. Euro
Surveill. 2021;26(50).
89.
European Centre for Disease Prevention and Control. Human papillomavirus infection:
recommended
vaccinations.
Available
from:
https://vaccine-
schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=38&SelectedCountryIdByDis
69
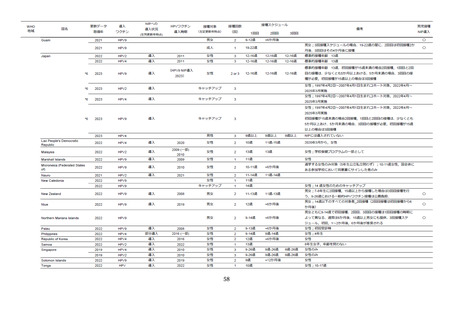
78.
Palmer C, Tobe K, Negishi Y, You X, Chen YT, Abe M. Health impact and cost
effectiveness of implementing gender-neutral HPV vaccination in Japan. J Med Econ.
2023;26(1):1546-54.
79.
Ng SS, Hutubessy R, Chaiyakunapruk N. Systematic review of cost-effectiveness studies
of human papillomavirus (HPV) vaccination: 9-Valent vaccine, gender-neutral and multiple age
cohort vaccination. Vaccine. 2018;36(19):2529-44.
80.
Cody P, Tobe K, Abe M, Elbasha EH. Public health impact and cost effectiveness of
routine and catch-up vaccination of girls and women with a nine-valent HPV vaccine in Japan: a
model-based study. BMC Infect Dis. 2021;21(1):11.
81.
Kitano T. Risk-Benefit Analysis of the 9-Valent HPV Vaccination for Adolescent Boys
from an Individual Perspective. Jpn J Infect Dis. 2022;75(2):114-20.
82.
Datta S, Pink J, Medley GF, Petrou S, Staniszewska S, Underwood M, et al. Assessing
the cost-effectiveness of HPV vaccination strategies for adolescent girls and boys in the UK. BMC
Infect Dis. 2019;19(1):552.
83.
WHO.
International
Agency
for
Research
on
Cancer.
Available
from:
https://gco.iarc.fr/today/online-analysis-table
84.
Ito Y, Miyashiro I, Ito H, Hosono S, Chihara D, Nakata-Yamada K, et al. Long-term
survival and conditional survival of cancer patients in Japan using population- based cancer
registry data. Cancer Sci. 2014;105(11):1480-6.
85.
Japan Society for Head and Neck Cancer Cancer Registry Committee. Report of Head
and Neck Cancer Registry of Japan Clinical Statistics of Registered Patients, 2020. Available from:
https://square.umin.ac.jp/~jshnc/pdf/HNCreport_2020.pdf
86.
Drolet M, Benard E, Perez N, Brisson M, Group HPVVIS. Population-level impact and
herd effects following the introduction of human papillomavirus vaccination programmes: updated
systematic review and meta-analysis. Lancet. 2019;394(10197):497-509.
87.
WHO.
HPV
Dashboard
2024.
Available
from:
https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/humanpapillomavirus-vaccines-(HPV)/hpv-clearing-house/hpv-dashboard.
88.
Colzani E, Johansen K, Johnson H, Pastore Celentano L. Human papillomavirus
vaccination in the European Union/European Economic Area and globally: a moral dilemma. Euro
Surveill. 2021;26(50).
89.
European Centre for Disease Prevention and Control. Human papillomavirus infection:
recommended
vaccinations.
Available
from:
https://vaccine-
schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=38&SelectedCountryIdByDis
69