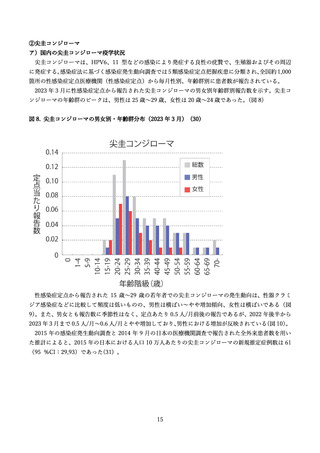
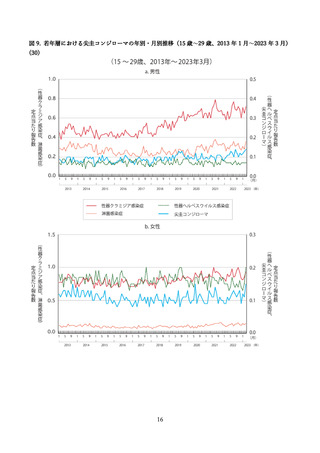
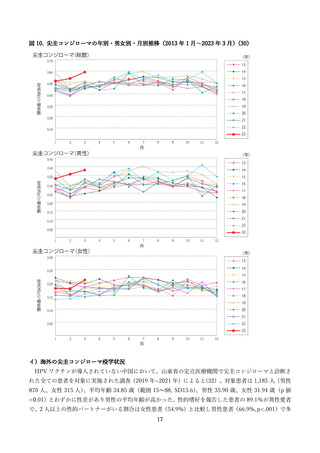
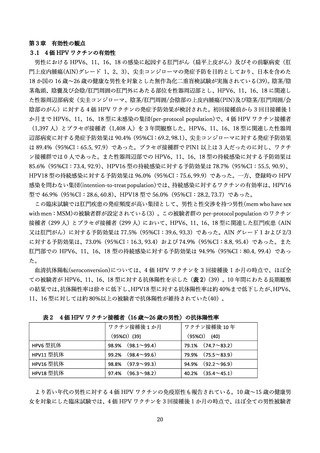
よむ、つかう、まなぶ。
08参考資料1 ヒトパピローマウイルス(HPV)ワクチンファクトシート追補版 (68 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_63875.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第31回 9/25)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
65.
Trials.gov C. A Phase III Study of a 2-dose Regimen of a Multivalent Human
Papillomavirus (HPV) Vaccine (V503), Administered to 9 to 14 Year-olds and Compared to
Young
Women,
16
to
26
Years
Old
(V503-010)
2018.
Available
from:
https://clinicaltrials.gov/ct2/show/results/NCT01984697#evnt
66.
独立行政法人医薬品医療機器総合機構. 組換え沈降 9 価ヒトパピローマウイルス様粒
子 ワ ク チ ン ( 酵 母 由 来 )
審 査 報 告 書 .
2023.
Available
from:
https://www.pmda.go.jp/drugs/2023/P20230306001/170050000_30200AMX00746_A100_1.pd
f
67.
Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, et al.
Immunogenicity of the 9-Valent HPV Vaccine Using 2-Dose Regimens in Girls and Boys vs a 3Dose Regimen in Women. JAMA. 2016;316(22):2411-21.
68.
Shimabukuro TT, Su JR, Marquez PL, Mba-Jonas A, Arana JE, Cano MV. Safety of the
9-Valent Human Papillomavirus Vaccine. Pediatrics. 2019;144(6).
69.
Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis
of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137(Pt 1):33-43.
70.
Bonaldo G, Montanaro N, Vaccheri A, Motola D. Human papilloma virus vaccination in
males: A pharmacovigilance study on the Vaccine Adverse Event Reporting System. Br J Clin
Pharmacol. 2021;87(4):1912-7.
71.
Donahue JG, Kieke BA, Lewis EM, Weintraub ES, Hanson KE, McClure DL, et al. Near
Real-Time Surveillance to Assess the Safety of the 9-Valent Human Papillomavirus Vaccine.
Pediatrics. 2019;144(6).
72.
Centers for Disease Control and Prevention. HPV Vaccine Safety and Effectiveness Data
2021. Available from: https://www.cdc.gov/hpv/hcp/vaccine-safety-data.html.
73.
Linertová R, Guirado-Fuentes C, Mar Medina J, Imaz-Iglesia I, Rodríguez-Rodríguez L,
Carmona-Rodríguez M. Cost-effectiveness of extending the HPV vaccination to boys: a systematic
review. J Epidemiol Community Health. 2021;75(9):910-6.
74.
Majed L, Bresse X, El Mouaddin N, Schmidt A, Daniels VJ, Pavelyev A, et al. Public
health impact and cost-effectiveness of a nine-valent gender-neutral HPV vaccination program in
France. Vaccine. 2021;39(2):438-46.
75.
Linertová R, Guirado-Fuentes C, Mar-Medina J, Teljeur C. Cost-effectiveness and
epidemiological impact of gender-neutral HPV vaccination in Spain. Hum Vaccin Immunother.
2022;18(6):2127983.
76.
Cheung TH, Cheng SSY, Hsu D, Wing-Lei Wong Q, Pavelyev A, Sukarom I, et al. Health
impact and cost-effectiveness of implementing gender-neutral vaccination with the 9-valent HPV
vaccine in Hong Kong. Hum Vaccin Immunother. 2023;19(2):2184605.
77.
Wahab MT, Tan RKJ, Cook AR, Prem K. Impact of including boys in the national school-
68
Trials.gov C. A Phase III Study of a 2-dose Regimen of a Multivalent Human
Papillomavirus (HPV) Vaccine (V503), Administered to 9 to 14 Year-olds and Compared to
Young
Women,
16
to
26
Years
Old
(V503-010)
2018.
Available
from:
https://clinicaltrials.gov/ct2/show/results/NCT01984697#evnt
66.
独立行政法人医薬品医療機器総合機構. 組換え沈降 9 価ヒトパピローマウイルス様粒
子 ワ ク チ ン ( 酵 母 由 来 )
審 査 報 告 書 .
2023.
Available
from:
https://www.pmda.go.jp/drugs/2023/P20230306001/170050000_30200AMX00746_A100_1.pd
f
67.
Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, et al.
Immunogenicity of the 9-Valent HPV Vaccine Using 2-Dose Regimens in Girls and Boys vs a 3Dose Regimen in Women. JAMA. 2016;316(22):2411-21.
68.
Shimabukuro TT, Su JR, Marquez PL, Mba-Jonas A, Arana JE, Cano MV. Safety of the
9-Valent Human Papillomavirus Vaccine. Pediatrics. 2019;144(6).
69.
Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis
of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137(Pt 1):33-43.
70.
Bonaldo G, Montanaro N, Vaccheri A, Motola D. Human papilloma virus vaccination in
males: A pharmacovigilance study on the Vaccine Adverse Event Reporting System. Br J Clin
Pharmacol. 2021;87(4):1912-7.
71.
Donahue JG, Kieke BA, Lewis EM, Weintraub ES, Hanson KE, McClure DL, et al. Near
Real-Time Surveillance to Assess the Safety of the 9-Valent Human Papillomavirus Vaccine.
Pediatrics. 2019;144(6).
72.
Centers for Disease Control and Prevention. HPV Vaccine Safety and Effectiveness Data
2021. Available from: https://www.cdc.gov/hpv/hcp/vaccine-safety-data.html.
73.
Linertová R, Guirado-Fuentes C, Mar Medina J, Imaz-Iglesia I, Rodríguez-Rodríguez L,
Carmona-Rodríguez M. Cost-effectiveness of extending the HPV vaccination to boys: a systematic
review. J Epidemiol Community Health. 2021;75(9):910-6.
74.
Majed L, Bresse X, El Mouaddin N, Schmidt A, Daniels VJ, Pavelyev A, et al. Public
health impact and cost-effectiveness of a nine-valent gender-neutral HPV vaccination program in
France. Vaccine. 2021;39(2):438-46.
75.
Linertová R, Guirado-Fuentes C, Mar-Medina J, Teljeur C. Cost-effectiveness and
epidemiological impact of gender-neutral HPV vaccination in Spain. Hum Vaccin Immunother.
2022;18(6):2127983.
76.
Cheung TH, Cheng SSY, Hsu D, Wing-Lei Wong Q, Pavelyev A, Sukarom I, et al. Health
impact and cost-effectiveness of implementing gender-neutral vaccination with the 9-valent HPV
vaccine in Hong Kong. Hum Vaccin Immunother. 2023;19(2):2184605.
77.
Wahab MT, Tan RKJ, Cook AR, Prem K. Impact of including boys in the national school-
68