よむ、つかう、まなぶ。
08参考資料1 ヒトパピローマウイルス(HPV)ワクチンファクトシート追補版 (67 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_63875.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第31回 9/25)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
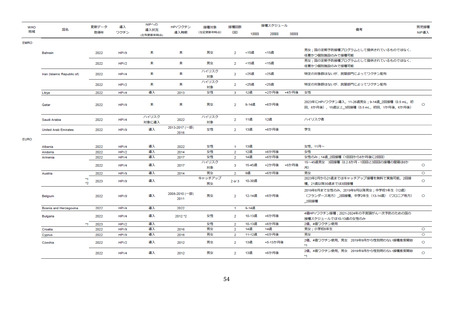
53.
Garland SM, Anagani M, Bhatla N, Chatterjee S, Lalwani S, Ross C, et al.
Immunogenicity and safety of quadrivalent and 9-valent human papillomavirus vaccines in Indian
clinical trial participants. Hum Vaccin Immunother. 2022;18(6):2105067.
54.
Widdice LE, Bernstein DI, Franco EL, Ding L, Brown DR, Ermel AC, et al. Decline in
vaccine-type human papillomavirus prevalence in young men from a Midwest metropolitan area
of the United States over the six years after vaccine introduction. Vaccine. 2019;37(45):6832-41.
55.
Chow EPF, Carter A, Vickers T, Fairley CK, McNulty A, Guy RJ, et al. Effect on genital
warts in Australian female and heterosexual male individuals after introduction of the national
human papillomavirus gender-neutral vaccination programme: an analysis of national sentinel
surveillance data from 2004-18. Lancet Infect Dis. 2021;21(12):1747-56.
56.
Chaturvedi AK, Graubard BI, Broutian T, Pickard RKL, Tong ZY, Xiao W, et al. Effect
of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young
Adults in the United States. J Clin Oncol. 2018;36(3):262-7.
57.
Hirth JM, Chang M, Resto VA. Prevalence of oral human papillomavirus by vaccination
status among young adults (18-30years old). Vaccine. 2017;35(27):3446-51.
58.
Schlecht NF, Masika M, Diaz A, Nucci-Sack A, Salandy A, Pickering S, et al. Risk of Oral
Human Papillomavirus Infection Among Sexually Active Female Adolescents Receiving the
Quadrivalent Vaccine. JAMA Netw Open. 2019;2(10):e1914031.
59.
Mehanna H, Bryant TS, Babrah J, Louie K, Bryant JL, Spruce RJ, et al. Human
Papillomavirus (HPV) Vaccine Effectiveness and Potential Herd Immunity for Reducing
Oncogenic Oropharyngeal HPV-16 Prevalence in the United Kingdom: A Cross-sectional Study.
Clin Infect Dis. 2019;69(8):1296-302.
60.
Ferris D, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Reisinger KS, et al.
Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics. 2014;134(3):e65765.
61.
Arana JE, Harrington T, Cano M, Lewis P, Mba-Jonas A, Rongxia L, et al. Post-licensure
safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event
Reporting System (VAERS), 2009-2015. Vaccine. 2018;36(13):1781-8.
62.
国立感染症研究所. 9価ヒトパピローマウイルス( HPV )ワクチンファクトシート
2021. Available from: https://www.mhlw.go.jp/content/10906000/000770615.pdf.
63.
Moreira ED, Giuliano AR, de Hoon J, Iversen OE, Joura EA, Restrepo J, et al. Safety
profile of the 9-valent human papillomavirus vaccine: assessment in prior quadrivalent HPV
vaccine recipients and in men 16 to 26 years of age. Hum Vaccin Immunother. 2018;14(2):396403.
64.
Bornstein J, Roux S, Kjeld Petersen L, Huang L-M, Dobson SR, Pitisuttithum P, et al.
Three-Year Follow-up of 2-Dose Versus 3-Dose HPV Vaccine. Pediatrics. 2021;147(1).
67
Garland SM, Anagani M, Bhatla N, Chatterjee S, Lalwani S, Ross C, et al.
Immunogenicity and safety of quadrivalent and 9-valent human papillomavirus vaccines in Indian
clinical trial participants. Hum Vaccin Immunother. 2022;18(6):2105067.
54.
Widdice LE, Bernstein DI, Franco EL, Ding L, Brown DR, Ermel AC, et al. Decline in
vaccine-type human papillomavirus prevalence in young men from a Midwest metropolitan area
of the United States over the six years after vaccine introduction. Vaccine. 2019;37(45):6832-41.
55.
Chow EPF, Carter A, Vickers T, Fairley CK, McNulty A, Guy RJ, et al. Effect on genital
warts in Australian female and heterosexual male individuals after introduction of the national
human papillomavirus gender-neutral vaccination programme: an analysis of national sentinel
surveillance data from 2004-18. Lancet Infect Dis. 2021;21(12):1747-56.
56.
Chaturvedi AK, Graubard BI, Broutian T, Pickard RKL, Tong ZY, Xiao W, et al. Effect
of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young
Adults in the United States. J Clin Oncol. 2018;36(3):262-7.
57.
Hirth JM, Chang M, Resto VA. Prevalence of oral human papillomavirus by vaccination
status among young adults (18-30years old). Vaccine. 2017;35(27):3446-51.
58.
Schlecht NF, Masika M, Diaz A, Nucci-Sack A, Salandy A, Pickering S, et al. Risk of Oral
Human Papillomavirus Infection Among Sexually Active Female Adolescents Receiving the
Quadrivalent Vaccine. JAMA Netw Open. 2019;2(10):e1914031.
59.
Mehanna H, Bryant TS, Babrah J, Louie K, Bryant JL, Spruce RJ, et al. Human
Papillomavirus (HPV) Vaccine Effectiveness and Potential Herd Immunity for Reducing
Oncogenic Oropharyngeal HPV-16 Prevalence in the United Kingdom: A Cross-sectional Study.
Clin Infect Dis. 2019;69(8):1296-302.
60.
Ferris D, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Reisinger KS, et al.
Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics. 2014;134(3):e65765.
61.
Arana JE, Harrington T, Cano M, Lewis P, Mba-Jonas A, Rongxia L, et al. Post-licensure
safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event
Reporting System (VAERS), 2009-2015. Vaccine. 2018;36(13):1781-8.
62.
国立感染症研究所. 9価ヒトパピローマウイルス( HPV )ワクチンファクトシート
2021. Available from: https://www.mhlw.go.jp/content/10906000/000770615.pdf.
63.
Moreira ED, Giuliano AR, de Hoon J, Iversen OE, Joura EA, Restrepo J, et al. Safety
profile of the 9-valent human papillomavirus vaccine: assessment in prior quadrivalent HPV
vaccine recipients and in men 16 to 26 years of age. Hum Vaccin Immunother. 2018;14(2):396403.
64.
Bornstein J, Roux S, Kjeld Petersen L, Huang L-M, Dobson SR, Pitisuttithum P, et al.
Three-Year Follow-up of 2-Dose Versus 3-Dose HPV Vaccine. Pediatrics. 2021;147(1).
67