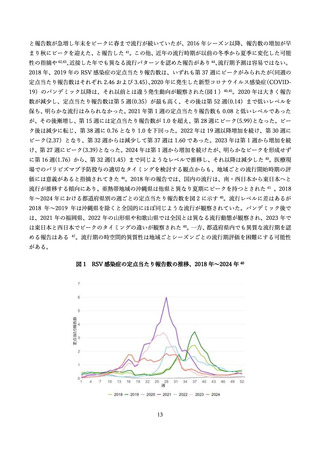
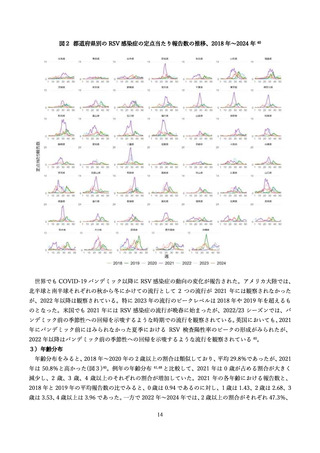
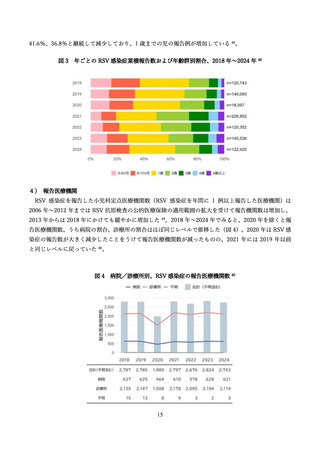
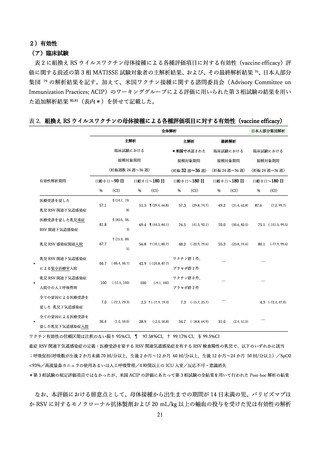
よむ、つかう、まなぶ。
05資料2-1森野委員提出資料(RSウイルス母子免疫ワクチンと抗体製剤ファクトシート) (52 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64997.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第32回 10/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
Syncytial Virus Vaccine in Protecting Infants from RSV Infection in Japan. Infect Dis Ther 2024; 13(7):
1665-82.
135.
Kieffer A, Beuvelet M, Moncayo G, et al. Disease Burden Associated with All Infants in Their
First RSV Season in the UK: A Static Model of Universal Immunization with Nirsevimab Against RSVRelated Outcomes. Infect Dis Ther 2024; 13(10): 2135-53.
136.
Rey-Ares L, Averin A, Zuccarino N, et al. Cost-Effectiveness of Bivalent Respiratory Syncytial
Virus Prefusion F (RSVpreF) Vaccine During Pregnancy for Prevention of Respiratory Syncytial Virus
Among Infants in Argentina. Infect Dis Ther 2024; 13(11): 2363-76.
137.
Yu T, Padula WV, Yieh L, Gong CL. Cost-effectiveness of nirsevimab and palivizumab for
respiratory syncytial virus prophylaxis in preterm infants 29-34 6/7 weeks' gestation in the United
States. Pediatr Neonatol 2024; 65(2): 152-8.
138.
Noto S, Kieffer A, Soudani S, et al. Cost-Effectiveness and Public Health Impact of Universal
Prophylaxis with Nirsevimab Against Respiratory Syncytial Virus (RSV) Infections in all Infants in
Japan. Infect Dis Ther 2025; 14(4): 847-65.
139.
Huerta JL, Kendall R, Ivkovic L, Molina C, Law AW, Mendes D. Economic and Clinical Benefits
of Bivalent Respiratory Syncytial Virus Prefusion F (RSVpreF) Maternal Vaccine for Prevention of RSV
in Infants: A Cost-Effectiveness Analysis for Mexico. Vaccines (Basel) 2025; 13(1).
140.
Nazareno AL, Wood JG, Muscatello DJ, Homaira N, Hogan AB, Newall AT. Estimating the cost-
effectiveness of maternal respiratory syncytial virus (RSV) vaccination in Australia: A dynamic and
economic modelling analysis. Vaccine 2025; 46: 126651.
141.
World Health Organization. WHO prequalifies first maternal respiratory syncytial virus
vaccine. 2025/3/19. https://www.who.int/news/item/19-03-2025-who-prequalifies-first-maternalrespiratory-syncytial-virus-vaccine (accessed 2025/05/02).
142.
World Health Organization. WHO Prequalification - Prequalified Vaccines: Abrysvo. 2025/3/12.
https://extranet.who.int/prequal/vaccines/p/abrysvo (accessed 2025/05/02).
143.
Haute Autorité de Santé(フランス高等保健機構). RSV infection vaccination recommendation
for pregnant women. 2024/10/14. https://has-sante.fr/jcms/p_3505344/en/rsv-infection-vaccinationrecommendation-for-pregnant-women (accessed 2025/10/06).
144.
Government of Canada. Summary Basis of Decision for Abrysvo -
Drug and Health Products
Portal. 2025/06/03. https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1715623184650/ (accessed
2025/10/06).
145.
European Medical Agency. Abrysvo. 2025/04/15.
https://www.ema.europa.eu/en/medicines/human/EPAR/abrysvo (accessed 2025/05/02).
146.
US Centers for Disease Control and Prevention. RSV Vaccine Guidance for Pregnant Women.
2024/08/30. https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/pregnant-people.html (accessed
2025/10/06).
147.
US Centers for Disease Control and Prevention. Respiratory Syncytial Virus Infection (RSV) -
Immunizations to Protect Infants. 2025/08/18. https://www.cdc.gov/rsv/vaccines/protect-infants.html
52
1665-82.
135.
Kieffer A, Beuvelet M, Moncayo G, et al. Disease Burden Associated with All Infants in Their
First RSV Season in the UK: A Static Model of Universal Immunization with Nirsevimab Against RSVRelated Outcomes. Infect Dis Ther 2024; 13(10): 2135-53.
136.
Rey-Ares L, Averin A, Zuccarino N, et al. Cost-Effectiveness of Bivalent Respiratory Syncytial
Virus Prefusion F (RSVpreF) Vaccine During Pregnancy for Prevention of Respiratory Syncytial Virus
Among Infants in Argentina. Infect Dis Ther 2024; 13(11): 2363-76.
137.
Yu T, Padula WV, Yieh L, Gong CL. Cost-effectiveness of nirsevimab and palivizumab for
respiratory syncytial virus prophylaxis in preterm infants 29-34 6/7 weeks' gestation in the United
States. Pediatr Neonatol 2024; 65(2): 152-8.
138.
Noto S, Kieffer A, Soudani S, et al. Cost-Effectiveness and Public Health Impact of Universal
Prophylaxis with Nirsevimab Against Respiratory Syncytial Virus (RSV) Infections in all Infants in
Japan. Infect Dis Ther 2025; 14(4): 847-65.
139.
Huerta JL, Kendall R, Ivkovic L, Molina C, Law AW, Mendes D. Economic and Clinical Benefits
of Bivalent Respiratory Syncytial Virus Prefusion F (RSVpreF) Maternal Vaccine for Prevention of RSV
in Infants: A Cost-Effectiveness Analysis for Mexico. Vaccines (Basel) 2025; 13(1).
140.
Nazareno AL, Wood JG, Muscatello DJ, Homaira N, Hogan AB, Newall AT. Estimating the cost-
effectiveness of maternal respiratory syncytial virus (RSV) vaccination in Australia: A dynamic and
economic modelling analysis. Vaccine 2025; 46: 126651.
141.
World Health Organization. WHO prequalifies first maternal respiratory syncytial virus
vaccine. 2025/3/19. https://www.who.int/news/item/19-03-2025-who-prequalifies-first-maternalrespiratory-syncytial-virus-vaccine (accessed 2025/05/02).
142.
World Health Organization. WHO Prequalification - Prequalified Vaccines: Abrysvo. 2025/3/12.
https://extranet.who.int/prequal/vaccines/p/abrysvo (accessed 2025/05/02).
143.
Haute Autorité de Santé(フランス高等保健機構). RSV infection vaccination recommendation
for pregnant women. 2024/10/14. https://has-sante.fr/jcms/p_3505344/en/rsv-infection-vaccinationrecommendation-for-pregnant-women (accessed 2025/10/06).
144.
Government of Canada. Summary Basis of Decision for Abrysvo -
Drug and Health Products
Portal. 2025/06/03. https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1715623184650/ (accessed
2025/10/06).
145.
European Medical Agency. Abrysvo. 2025/04/15.
https://www.ema.europa.eu/en/medicines/human/EPAR/abrysvo (accessed 2025/05/02).
146.
US Centers for Disease Control and Prevention. RSV Vaccine Guidance for Pregnant Women.
2024/08/30. https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/pregnant-people.html (accessed
2025/10/06).
147.
US Centers for Disease Control and Prevention. Respiratory Syncytial Virus Infection (RSV) -
Immunizations to Protect Infants. 2025/08/18. https://www.cdc.gov/rsv/vaccines/protect-infants.html
52