よむ、つかう、まなぶ。
05資料2-1森野委員提出資料(RSウイルス母子免疫ワクチンと抗体製剤ファクトシート) (49 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64997.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第32回 10/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
91.
Wilkins D, Langedijk AC, Lebbink RJ, et al. Nirsevimab binding-site conservation in respiratory
syncytial virus fusion glycoprotein worldwide between 1956 and 2021: an analysis of observational study
sequencing data. Lancet Infect Dis 2023; 23(7): 856-66.
92.
Zhu Q, Lu B, McTamney P, et al. Prevalence and Significance of Substitutions in the Fusion
Protein of Respiratory Syncytial Virus Resulting in Neutralization Escape From Antibody MEDI8897. J
Infect Dis 2018; 218(4): 572-80.
93.
Ahani B, Tuffy KM, Aksyuk AA, et al. Molecular and phenotypic characteristics of RSV
infections in infants during two nirsevimab randomized clinical trials. Nat Commun 2023; 14(1): 4347.
94.
Ares-Gómez S, Mallah N, Santiago-Pérez MI, et al. Effectiveness and impact of universal
prophylaxis with nirsevimab in infants against hospitalisation for respiratory syncytial virus in Galicia,
Spain: initial results of a population-based longitudinal study. Lancet Infect Dis 2024; 24(8): 817-28.
95.
Lassoued Y, Levy C, Werner A, et al. Effectiveness of nirsevimab against RSV-bronchiolitis in
paediatric ambulatory care: a test-negative case-control study. Lancet Reg Health Eur 2024; 44: 101007.
96.
Group TI-RS. Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody,
Reduces Hospitalization From Respiratory Syncytial Virus Infection in High-risk Infants. Pediatrics
1998; 102(3): 531-7.
97.
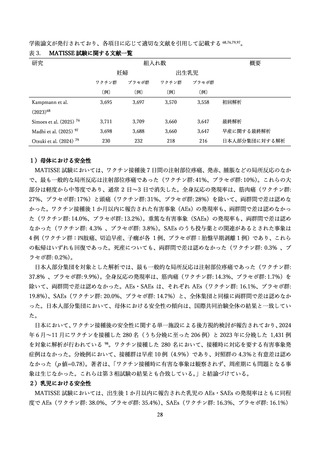
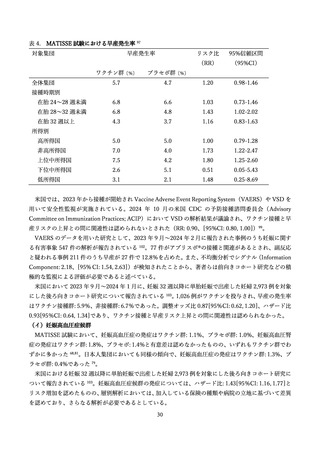
Madhi SA, Kampmann B, Simoes EAF, et al. Preterm Birth Frequency and Associated
Outcomes From the MATISSE (Maternal Immunization Study for Safety and Efficacy) Maternal Trial of
the Bivalent Respiratory Syncytial Virus Prefusion F Protein Vaccine. Obstet Gynecol 2025; 145(2): 14756.
98.
山中美智子. RS ウイルス母子免疫ワクチンの接種実績報告. 日本周産期・新生児医学会雑誌 2025;
61(1).
99.
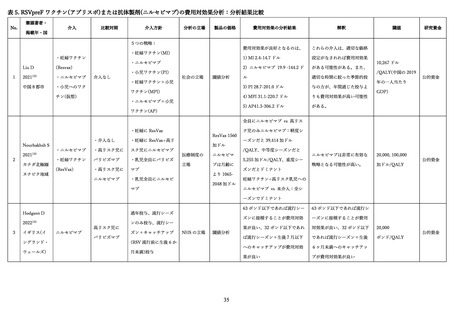
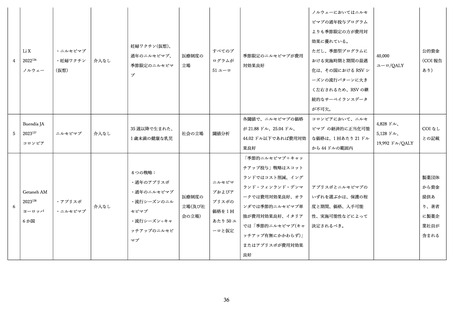
DeSilva M. RSVpreF Vaccine, Preterm Birth, and Small for Gestational Age at Birth
Preliminary Results from The Vaccine Safety Datalink. ACIP presentation slide 2024.
100.
US Food and Drug Administration. FDA Briefing Document Respiratory Syncytial Virus
Vaccine (Proposed Trade Name: Abrysvo). 2023.
101.
European Medicines Agency. Assessment report Abrysvo International non-proprietary name:
respiratory syncytial virus vaccine (bivalent, recombinant). 2023.
102.
Alami A, Perez-Lloret S, Mattison DR. Safety surveillance of respiratory syncytial virus (RSV)
vaccine among pregnant individuals: a real-world pharmacovigilance study using the Vaccine Adverse
Event Reporting System. BMJ Open 2025; 15(4): e087850.
103.
Son M, Riley LE, Staniczenko AP, et al. Nonadjuvanted Bivalent Respiratory Syncytial Virus
Vaccination and Perinatal Outcomes. JAMA Netw Open 2024; 7(7): e2419268.
104.
Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite
prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89(4): 422-34.
105.
Acosta PL, Caballero MT, Polack FP. Brief History and Characterization of Enhanced
Respiratory Syncytial Virus Disease. Clin Vaccine Immunol 2015; 23(3): 189-95.
106.
Graham BS. Biological challenges and technological opportunities for respiratory syncytial virus
49
Wilkins D, Langedijk AC, Lebbink RJ, et al. Nirsevimab binding-site conservation in respiratory
syncytial virus fusion glycoprotein worldwide between 1956 and 2021: an analysis of observational study
sequencing data. Lancet Infect Dis 2023; 23(7): 856-66.
92.
Zhu Q, Lu B, McTamney P, et al. Prevalence and Significance of Substitutions in the Fusion
Protein of Respiratory Syncytial Virus Resulting in Neutralization Escape From Antibody MEDI8897. J
Infect Dis 2018; 218(4): 572-80.
93.
Ahani B, Tuffy KM, Aksyuk AA, et al. Molecular and phenotypic characteristics of RSV
infections in infants during two nirsevimab randomized clinical trials. Nat Commun 2023; 14(1): 4347.
94.
Ares-Gómez S, Mallah N, Santiago-Pérez MI, et al. Effectiveness and impact of universal
prophylaxis with nirsevimab in infants against hospitalisation for respiratory syncytial virus in Galicia,
Spain: initial results of a population-based longitudinal study. Lancet Infect Dis 2024; 24(8): 817-28.
95.
Lassoued Y, Levy C, Werner A, et al. Effectiveness of nirsevimab against RSV-bronchiolitis in
paediatric ambulatory care: a test-negative case-control study. Lancet Reg Health Eur 2024; 44: 101007.
96.
Group TI-RS. Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody,
Reduces Hospitalization From Respiratory Syncytial Virus Infection in High-risk Infants. Pediatrics
1998; 102(3): 531-7.
97.
Madhi SA, Kampmann B, Simoes EAF, et al. Preterm Birth Frequency and Associated
Outcomes From the MATISSE (Maternal Immunization Study for Safety and Efficacy) Maternal Trial of
the Bivalent Respiratory Syncytial Virus Prefusion F Protein Vaccine. Obstet Gynecol 2025; 145(2): 14756.
98.
山中美智子. RS ウイルス母子免疫ワクチンの接種実績報告. 日本周産期・新生児医学会雑誌 2025;
61(1).
99.
DeSilva M. RSVpreF Vaccine, Preterm Birth, and Small for Gestational Age at Birth
Preliminary Results from The Vaccine Safety Datalink. ACIP presentation slide 2024.
100.
US Food and Drug Administration. FDA Briefing Document Respiratory Syncytial Virus
Vaccine (Proposed Trade Name: Abrysvo). 2023.
101.
European Medicines Agency. Assessment report Abrysvo International non-proprietary name:
respiratory syncytial virus vaccine (bivalent, recombinant). 2023.
102.
Alami A, Perez-Lloret S, Mattison DR. Safety surveillance of respiratory syncytial virus (RSV)
vaccine among pregnant individuals: a real-world pharmacovigilance study using the Vaccine Adverse
Event Reporting System. BMJ Open 2025; 15(4): e087850.
103.
Son M, Riley LE, Staniczenko AP, et al. Nonadjuvanted Bivalent Respiratory Syncytial Virus
Vaccination and Perinatal Outcomes. JAMA Netw Open 2024; 7(7): e2419268.
104.
Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite
prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89(4): 422-34.
105.
Acosta PL, Caballero MT, Polack FP. Brief History and Characterization of Enhanced
Respiratory Syncytial Virus Disease. Clin Vaccine Immunol 2015; 23(3): 189-95.
106.
Graham BS. Biological challenges and technological opportunities for respiratory syncytial virus
49