よむ、つかう、まなぶ。
05資料2-1森野委員提出資料(RSウイルス母子免疫ワクチンと抗体製剤ファクトシート) (50 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64997.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第32回 10/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
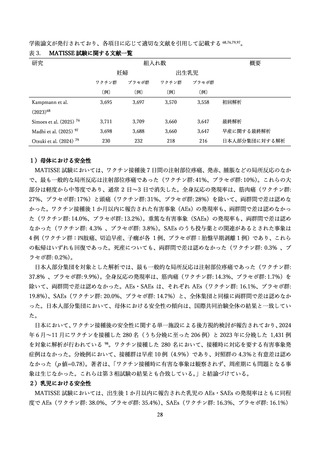
vaccine development. Immunol Rev 2011; 239(1): 149-66.
107.
Razzaghi H, Garacci E, Kahn KE, et al. Maternal Respiratory Syncytial Virus Vaccination and
Receipt of Respiratory Syncytial Virus Antibody (Nirsevimab) by Infants Aged <8 Months - United
States, April 2024. MMWR Morb Mortal Wkly Rep 2024; 73(38): 837-43.
108.
US Centers for Disease Control and Prevention. Respiratory Syncytial Virus (RSV) Vaccine
Safety. CDC Vaccine Safety website 2025.
109.
US Food and Drug Administration. FDA Requires Guillain-Barré Syndrome (GBS) Warning in
the Prescribing Information for RSV Vaccines Abrysvo and Arexvy. 2025.
110.
Lloyd P. Evaluation of Guillain-Barré Syndrome (GBS) following Respiratory Syncytial Virus
(RSV) Vaccination Among Adults 65 Years and Older. ACIP presentation slide 2024.
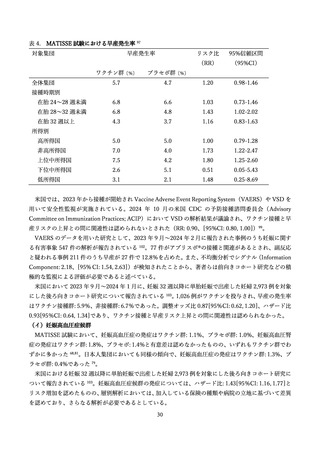
111.
Britton A, Roper LE, Kotton CN, et al. Use of Respiratory Syncytial Virus Vaccines in Adults
Aged >/=60 Years: Updated Recommendations of the Advisory Committee on Immunization Practices United States, 2024. MMWR Morb Mortal Wkly Rep 2024; 73(32): 696-702.
112.
de Bruin O, Nab L, Choi J, et al. A Post-Authorisation Safety Study of a Respiratory Syncytial
Virus Vaccine in Pregnant Women and Their Offspring in a Real-World Setting: Generic Protocol for a
Target Trial Emulation. Vaccines (Basel) 2025; 13(3).
113.
Jones J. Maternal/Pediatric RSV Work Group Considerations. ACIP presentation slide 2024.
114.
GOV.UK. UK. RSV vaccination of pregnant women for infant protection: information for
healthcare practitioners. Published July 12, 2024 2025.
https://www.gov.uk/government/publications/respiratory-syncytial-virus-rsv-programme-information-forhealthcare-professionals/rsv-vaccination-of-pregnant-women-for-infant-protection-information-forhealthcare-practitioners (accessed 2025/7/31).
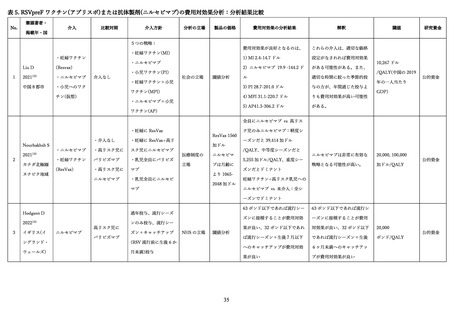
115.
Gonzales T, Bergamasco A, Cristarella T, et al. Effectiveness and Safety of Palivizumab for the
Prevention of Serious Lower Respiratory Tract Infection Caused by Respiratory Syncytial Virus: A
Systematic Review. Am J Perinatol 2024; 41(S 01): e1107-e15.
116.
Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of Nirsevimab for the Prevention of
Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the
Advisory Committee on Immunization Practices - United States, 2023. MMWR Morb Mortal Wkly Rep
2023; 72(34): 920-5.
117.
Esposito S, Abu-Raya B, Bonanni P, et al. Coadministration of Anti-Viral Monoclonal Antibodies
With Routine Pediatric Vaccines and Implications for Nirsevimab Use: A White Paper. Front Immunol
2021; 12: 708939.
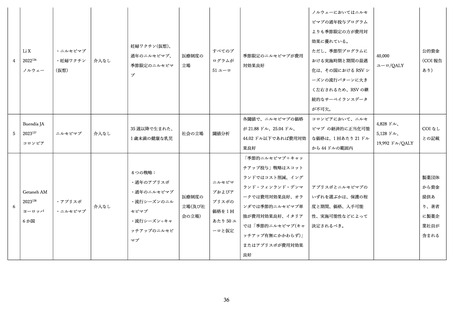
118.
US Department of Health and Human Services CDC. General best practice guidelines for
immunization. 2023. https://www.cdc.gov/vaccines/hcp/imz-best-practices/index.html (accessed
2025/03/24).
119.
Domachowske J, Madhi SA, Simões EAF, et al. Safety of Nirsevimab for RSV in Infants with
Heart or Lung Disease or Prematurity. N Engl J Med 2022; 386(9): 892-4.
120.
Domachowske JB, Chang Y, Atanasova V, et al. Safety of Re-dosing Nirsevimab Prior to RSV
50
107.
Razzaghi H, Garacci E, Kahn KE, et al. Maternal Respiratory Syncytial Virus Vaccination and
Receipt of Respiratory Syncytial Virus Antibody (Nirsevimab) by Infants Aged <8 Months - United
States, April 2024. MMWR Morb Mortal Wkly Rep 2024; 73(38): 837-43.
108.
US Centers for Disease Control and Prevention. Respiratory Syncytial Virus (RSV) Vaccine
Safety. CDC Vaccine Safety website 2025.
109.
US Food and Drug Administration. FDA Requires Guillain-Barré Syndrome (GBS) Warning in
the Prescribing Information for RSV Vaccines Abrysvo and Arexvy. 2025.
110.
Lloyd P. Evaluation of Guillain-Barré Syndrome (GBS) following Respiratory Syncytial Virus
(RSV) Vaccination Among Adults 65 Years and Older. ACIP presentation slide 2024.
111.
Britton A, Roper LE, Kotton CN, et al. Use of Respiratory Syncytial Virus Vaccines in Adults
Aged >/=60 Years: Updated Recommendations of the Advisory Committee on Immunization Practices United States, 2024. MMWR Morb Mortal Wkly Rep 2024; 73(32): 696-702.
112.
de Bruin O, Nab L, Choi J, et al. A Post-Authorisation Safety Study of a Respiratory Syncytial
Virus Vaccine in Pregnant Women and Their Offspring in a Real-World Setting: Generic Protocol for a
Target Trial Emulation. Vaccines (Basel) 2025; 13(3).
113.
Jones J. Maternal/Pediatric RSV Work Group Considerations. ACIP presentation slide 2024.
114.
GOV.UK. UK. RSV vaccination of pregnant women for infant protection: information for
healthcare practitioners. Published July 12, 2024 2025.
https://www.gov.uk/government/publications/respiratory-syncytial-virus-rsv-programme-information-forhealthcare-professionals/rsv-vaccination-of-pregnant-women-for-infant-protection-information-forhealthcare-practitioners (accessed 2025/7/31).
115.
Gonzales T, Bergamasco A, Cristarella T, et al. Effectiveness and Safety of Palivizumab for the
Prevention of Serious Lower Respiratory Tract Infection Caused by Respiratory Syncytial Virus: A
Systematic Review. Am J Perinatol 2024; 41(S 01): e1107-e15.
116.
Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of Nirsevimab for the Prevention of
Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the
Advisory Committee on Immunization Practices - United States, 2023. MMWR Morb Mortal Wkly Rep
2023; 72(34): 920-5.
117.
Esposito S, Abu-Raya B, Bonanni P, et al. Coadministration of Anti-Viral Monoclonal Antibodies
With Routine Pediatric Vaccines and Implications for Nirsevimab Use: A White Paper. Front Immunol
2021; 12: 708939.
118.
US Department of Health and Human Services CDC. General best practice guidelines for
immunization. 2023. https://www.cdc.gov/vaccines/hcp/imz-best-practices/index.html (accessed
2025/03/24).
119.
Domachowske J, Madhi SA, Simões EAF, et al. Safety of Nirsevimab for RSV in Infants with
Heart or Lung Disease or Prematurity. N Engl J Med 2022; 386(9): 892-4.
120.
Domachowske JB, Chang Y, Atanasova V, et al. Safety of Re-dosing Nirsevimab Prior to RSV
50