よむ、つかう、まなぶ。
05資料2-1森野委員提出資料(RSウイルス母子免疫ワクチンと抗体製剤ファクトシート) (51 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64997.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第32回 10/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
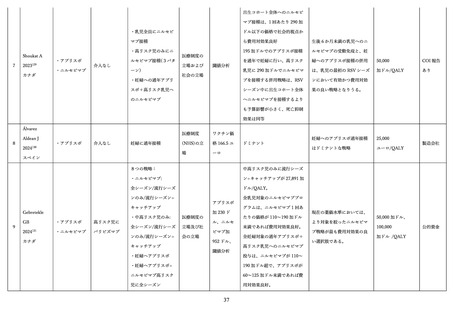
Season 2 in Children With Heart or Lung Disease. J Pediatric Infect Dis Soc 2023; 12(8): 477-80.
121.
Domachowske J, Hamrén UW, Banu I, et al. Safety and Pharmacokinetics of Nirsevimab in
Immunocompromised Children. Pediatrics 2024; 154(4).
122.
Carcione D, Spencer P, Pettigrew G, et al. ACTIVE POST-MARKETING SAFETY
SURVEILLANCE OF NIRSEVIMAB ADMINISTERED TO CHILDREN IN WESTERN AUSTRALIA,
APRIL-JULY 2024. Pediatr Infect Dis J 2025.
123.
Liu D, Leung K, Jit M, Wu JT. Cost-effectiveness of strategies for preventing paediatric lower
respiratory infections associated with respiratory syncytial virus in eight Chinese cities. Vaccine 2021;
39(39): 5490-8.
124.
Nourbakhsh S, Shoukat A, Zhang K, et al. Effectiveness and cost-effectiveness of RSV infant
and maternal immunization programs: A case study of Nunavik, Canada. EClinicalMedicine 2021; 41:
101141.
125.
Hodgson D, Koltai M, Krauer F, Flasche S, Jit M, Atkins KE. Optimal Respiratory Syncytial
Virus intervention programmes using Nirsevimab in England and Wales. Vaccine 2022; 40(49): 7151-7.
126.
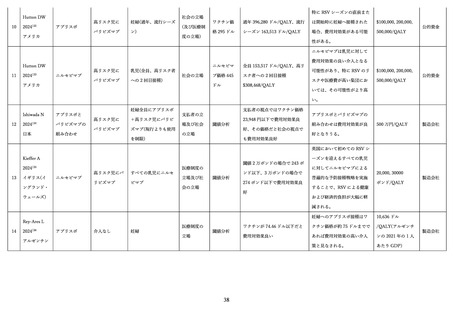
Li X, Bilcke J, Vázquez Fernández L, et al. Cost-effectiveness of Respiratory Syncytial Virus
Disease Prevention Strategies: Maternal Vaccine Versus Seasonal or Year-Round Monoclonal Antibody
Program in Norwegian Children. J Infect Dis 2022; 226(Suppl 1): S95-s101.
127.
Buendía JA, Acuña-Cordero R, Rodriguez-Martinez CE. Exploratory analysis of the
economically justifiable price of nirsevimab for healthy late-preterm and term infants in Colombia.
Pediatr Pulmonol 2024; 59(5): 1372-9.
128.
Getaneh AM, Li X, Mao Z, et al. Cost-effectiveness of monoclonal antibody and maternal
immunization against respiratory syncytial virus (RSV) in infants: Evaluation for six European
countries. Vaccine 2023; 41(9): 1623-31.
129.
Shoukat A, Abdollahi E, Galvani AP, Halperin SA, Langley JM, Moghadas SM. Cost-
effectiveness analysis of nirsevimab and maternal RSVpreF vaccine strategies for prevention of
Respiratory Syncytial Virus disease among infants in Canada: a simulation study. Lancet Reg Health Am
2023; 28: 100629.
130.
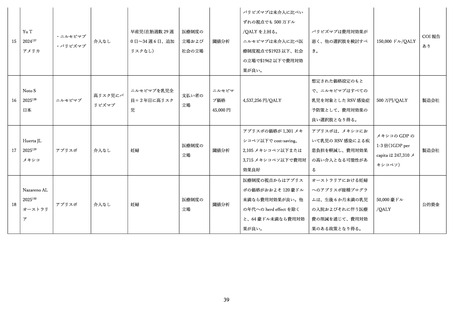
Álvarez Aldean J, Rivero Calle I, Rodríguez Fernández R, et al. Cost-effectiveness Analysis of
Maternal Immunization with RSVpreF Vaccine for the Prevention of Respiratory Syncytial Virus Among
Infants in Spain. Infect Dis Ther 2024; 13(6): 1315-31.
131.
Gebretekle GB, Yeung MW, Ximenes R, et al. Cost-effectiveness of RSVpreF vaccine and
nirsevimab for the prevention of respiratory syncytial virus disease in Canadian infants. Vaccine 2024;
42(21): 126164.
132.
Hutton DW, Prosser LA, Rose AM, et al. Cost-Effectiveness of Maternal Vaccination to Prevent
Respiratory Syncytial Virus Illness. Pediatrics 2024; 154(6).
133.
Hutton DW, Prosser LA, Rose AM, et al. Cost-Effectiveness of Nirsevimab for Respiratory
Syncytial Virus in Infants and Young Children. Pediatrics 2024; 154(6).
134.
Ishiwada N, Akaishi R, Kobayashi Y, et al. Cost-Effectiveness Analysis of Maternal Respiratory
51
121.
Domachowske J, Hamrén UW, Banu I, et al. Safety and Pharmacokinetics of Nirsevimab in
Immunocompromised Children. Pediatrics 2024; 154(4).
122.
Carcione D, Spencer P, Pettigrew G, et al. ACTIVE POST-MARKETING SAFETY
SURVEILLANCE OF NIRSEVIMAB ADMINISTERED TO CHILDREN IN WESTERN AUSTRALIA,
APRIL-JULY 2024. Pediatr Infect Dis J 2025.
123.
Liu D, Leung K, Jit M, Wu JT. Cost-effectiveness of strategies for preventing paediatric lower
respiratory infections associated with respiratory syncytial virus in eight Chinese cities. Vaccine 2021;
39(39): 5490-8.
124.
Nourbakhsh S, Shoukat A, Zhang K, et al. Effectiveness and cost-effectiveness of RSV infant
and maternal immunization programs: A case study of Nunavik, Canada. EClinicalMedicine 2021; 41:
101141.
125.
Hodgson D, Koltai M, Krauer F, Flasche S, Jit M, Atkins KE. Optimal Respiratory Syncytial
Virus intervention programmes using Nirsevimab in England and Wales. Vaccine 2022; 40(49): 7151-7.
126.
Li X, Bilcke J, Vázquez Fernández L, et al. Cost-effectiveness of Respiratory Syncytial Virus
Disease Prevention Strategies: Maternal Vaccine Versus Seasonal or Year-Round Monoclonal Antibody
Program in Norwegian Children. J Infect Dis 2022; 226(Suppl 1): S95-s101.
127.
Buendía JA, Acuña-Cordero R, Rodriguez-Martinez CE. Exploratory analysis of the
economically justifiable price of nirsevimab for healthy late-preterm and term infants in Colombia.
Pediatr Pulmonol 2024; 59(5): 1372-9.
128.
Getaneh AM, Li X, Mao Z, et al. Cost-effectiveness of monoclonal antibody and maternal
immunization against respiratory syncytial virus (RSV) in infants: Evaluation for six European
countries. Vaccine 2023; 41(9): 1623-31.
129.
Shoukat A, Abdollahi E, Galvani AP, Halperin SA, Langley JM, Moghadas SM. Cost-
effectiveness analysis of nirsevimab and maternal RSVpreF vaccine strategies for prevention of
Respiratory Syncytial Virus disease among infants in Canada: a simulation study. Lancet Reg Health Am
2023; 28: 100629.
130.
Álvarez Aldean J, Rivero Calle I, Rodríguez Fernández R, et al. Cost-effectiveness Analysis of
Maternal Immunization with RSVpreF Vaccine for the Prevention of Respiratory Syncytial Virus Among
Infants in Spain. Infect Dis Ther 2024; 13(6): 1315-31.
131.
Gebretekle GB, Yeung MW, Ximenes R, et al. Cost-effectiveness of RSVpreF vaccine and
nirsevimab for the prevention of respiratory syncytial virus disease in Canadian infants. Vaccine 2024;
42(21): 126164.
132.
Hutton DW, Prosser LA, Rose AM, et al. Cost-Effectiveness of Maternal Vaccination to Prevent
Respiratory Syncytial Virus Illness. Pediatrics 2024; 154(6).
133.
Hutton DW, Prosser LA, Rose AM, et al. Cost-Effectiveness of Nirsevimab for Respiratory
Syncytial Virus in Infants and Young Children. Pediatrics 2024; 154(6).
134.
Ishiwada N, Akaishi R, Kobayashi Y, et al. Cost-Effectiveness Analysis of Maternal Respiratory
51