よむ、つかう、まなぶ。
【参考資料2-2】抗微生物薬適正使用の手引き 第四版(案)医科・入院編 (51 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64503.html |
| 出典情報 | 厚生科学審議会 感染症部会(第99回 10/21)《厚生労働省》 |
ページ画像
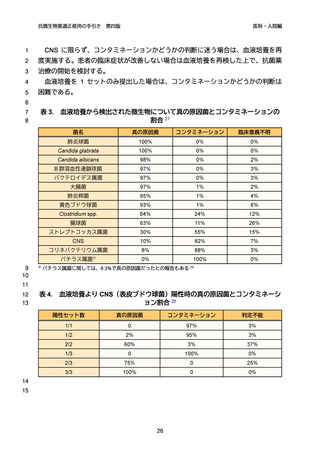
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
抗微生物薬適正使用の手引き
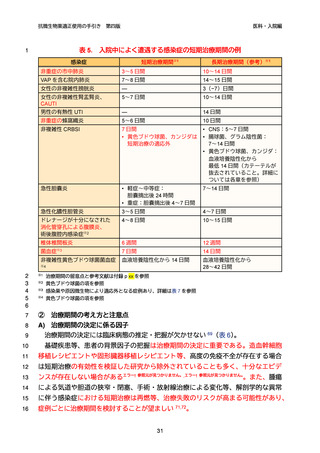
第四版
医科・入院編
1
7. 引用文献
2
3
4
1.
Bennett JE, et al. Mandell, Douglas, and Bennett's principles and practice of infectious
diseases. 9th ed. Philadelphia: Elsevier. 2019.
5
6
2.
Tamma PD, et al. Association of Adverse Events With Antibiotic Use in Hospitalized
Patients. JAMA Intern Med. 2017. 177(9):1308-1315.
7
8
3.
Gurtler N, et al. Appropriateness of antimicrobial prescribing in a Swiss tertiary care
hospital: a repeated point prevalence survey. Swiss Med Wkly. 2019. 149:w20135.
9
10
11
4.
Komagamine J, et al. Prevalence of antimicrobial use and active healthcare-associated
infections in acute care hospitals: a multicentre prevalence survey in Japan. BMJ Open.
2019. 9(6):e027604.
12
13
14
15
16
17
5.
el Moussaoui R, et al. Effectiveness of discontinuing antibiotic treatment after three days
versus eight days in mild to moderate-severe community acquired pneumonia:
randomised, double blind study. BMJ. 2006. 332(7554):1355.File TM Jr, et al.
Gemifloxacin once daily for 5 days versus 7 days for the treatment of communityacquired pneumonia: a randomized, multicentre, double-blind study. J Antimicrob
Chemother. 2007. 60(1):112-20.
18
19
6.
Uranga A, et al. Duration of Antibiotic Treatment in Community-Acquired Pneumonia: A
Multicenter Randomized Clinical Trial. JAMA Intern Med. 2016. 176(9):1257-65.
20
21
22
23
7.
Metlay JP, et al. Diagnosis and Treatment of Adults with Community-acquired
Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and
Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019. 200(7):e45e67.
24
25
26
8.
Pernica JM, et al. Short-Course Antimicrobial Therapy for Pediatric Community-Acquired
Pneumonia: The SAFER Randomized Clinical Trial. JAMA Pediatr. 2021. 175(5):475482.
27
28
29
30
9.
Dinh A, et al. Pneumonia Short Treatment (PTC) Study Group. Discontinuing β-lactam
treatment after 3 days for patients with community-acquired pneumonia in non-critical
care wards (PTC): a double-blind, randomised, placebo-controlled, non-inferiority trial.
Lancet. 2021.397(10280):1195-1203. Erratum in: Lancet. 2021. 397(10290):2150.
31
32
33
34
10. Bielicki JA, et al. Effect of Amoxicillin Dose and Treatment Duration on the Need for
Antibiotic Re-treatment in Children With Community-Acquired Pneumonia: The CAP-IT
Randomized Clinical Trial. JAMA. 2021. 326(17):1713-1724. Erratum in: JAMA. 2021.
326(21):2208.
35
36
37
11. Li Q, et al. Short-Course vs Long-Course Antibiotic Therapy for Children With Nonsevere
Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. JAMA
Pediatr. 2022. 176(12):1199-1207.
38
39
40
12. Furukawa Y, et al. Optimal duration of antibiotic treatment for community-acquired
pneumonia in adults: a systematic review and duration-effect meta-analysis. BMJ Open.
2023. 13(3):e061023.
41
42
43
13. Bougle A, et al. Comparison of 8 versus 15 days of antibiotic therapy for Pseudomonas
aeruginosa ventilator-associated pneumonia in adults: a randomized, controlled, openlabel trial. Intensive Care Med. 2022. 48(7):841-849.
51
第四版
医科・入院編
1
7. 引用文献
2
3
4
1.
Bennett JE, et al. Mandell, Douglas, and Bennett's principles and practice of infectious
diseases. 9th ed. Philadelphia: Elsevier. 2019.
5
6
2.
Tamma PD, et al. Association of Adverse Events With Antibiotic Use in Hospitalized
Patients. JAMA Intern Med. 2017. 177(9):1308-1315.
7
8
3.
Gurtler N, et al. Appropriateness of antimicrobial prescribing in a Swiss tertiary care
hospital: a repeated point prevalence survey. Swiss Med Wkly. 2019. 149:w20135.
9
10
11
4.
Komagamine J, et al. Prevalence of antimicrobial use and active healthcare-associated
infections in acute care hospitals: a multicentre prevalence survey in Japan. BMJ Open.
2019. 9(6):e027604.
12
13
14
15
16
17
5.
el Moussaoui R, et al. Effectiveness of discontinuing antibiotic treatment after three days
versus eight days in mild to moderate-severe community acquired pneumonia:
randomised, double blind study. BMJ. 2006. 332(7554):1355.File TM Jr, et al.
Gemifloxacin once daily for 5 days versus 7 days for the treatment of communityacquired pneumonia: a randomized, multicentre, double-blind study. J Antimicrob
Chemother. 2007. 60(1):112-20.
18
19
6.
Uranga A, et al. Duration of Antibiotic Treatment in Community-Acquired Pneumonia: A
Multicenter Randomized Clinical Trial. JAMA Intern Med. 2016. 176(9):1257-65.
20
21
22
23
7.
Metlay JP, et al. Diagnosis and Treatment of Adults with Community-acquired
Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and
Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019. 200(7):e45e67.
24
25
26
8.
Pernica JM, et al. Short-Course Antimicrobial Therapy for Pediatric Community-Acquired
Pneumonia: The SAFER Randomized Clinical Trial. JAMA Pediatr. 2021. 175(5):475482.
27
28
29
30
9.
Dinh A, et al. Pneumonia Short Treatment (PTC) Study Group. Discontinuing β-lactam
treatment after 3 days for patients with community-acquired pneumonia in non-critical
care wards (PTC): a double-blind, randomised, placebo-controlled, non-inferiority trial.
Lancet. 2021.397(10280):1195-1203. Erratum in: Lancet. 2021. 397(10290):2150.
31
32
33
34
10. Bielicki JA, et al. Effect of Amoxicillin Dose and Treatment Duration on the Need for
Antibiotic Re-treatment in Children With Community-Acquired Pneumonia: The CAP-IT
Randomized Clinical Trial. JAMA. 2021. 326(17):1713-1724. Erratum in: JAMA. 2021.
326(21):2208.
35
36
37
11. Li Q, et al. Short-Course vs Long-Course Antibiotic Therapy for Children With Nonsevere
Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. JAMA
Pediatr. 2022. 176(12):1199-1207.
38
39
40
12. Furukawa Y, et al. Optimal duration of antibiotic treatment for community-acquired
pneumonia in adults: a systematic review and duration-effect meta-analysis. BMJ Open.
2023. 13(3):e061023.
41
42
43
13. Bougle A, et al. Comparison of 8 versus 15 days of antibiotic therapy for Pseudomonas
aeruginosa ventilator-associated pneumonia in adults: a randomized, controlled, openlabel trial. Intensive Care Med. 2022. 48(7):841-849.
51