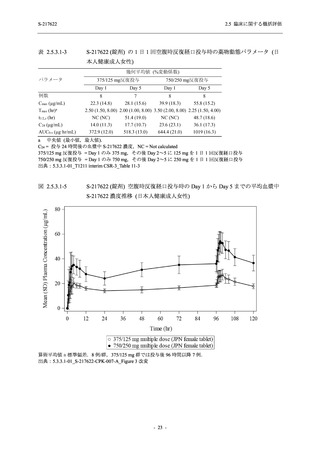
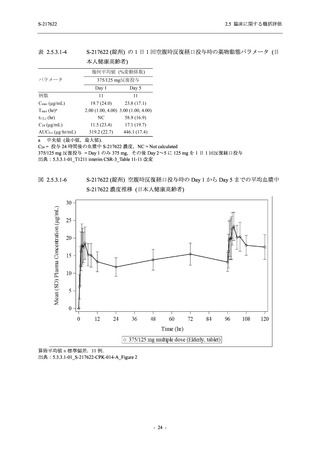
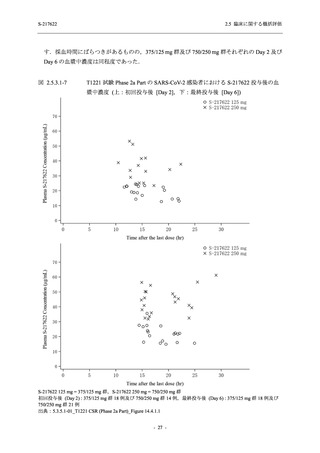
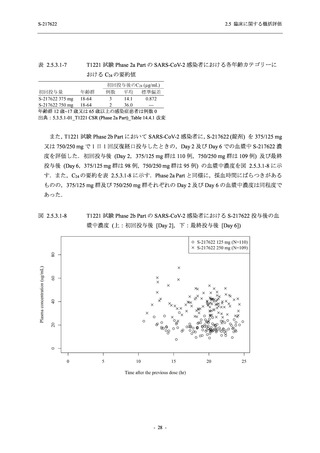
【資料No.1】2.5_臨床に関する概括資料 (131 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_26901.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会(令和4年度第3回 7/20)、医薬品第二部会(令和4年度第6回 7/20)(合同開催)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
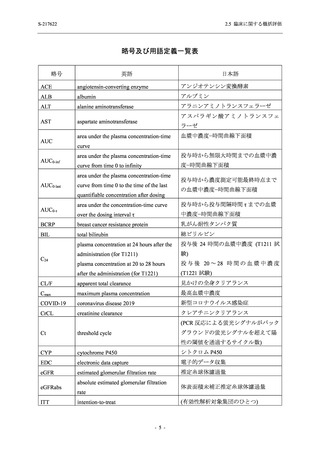
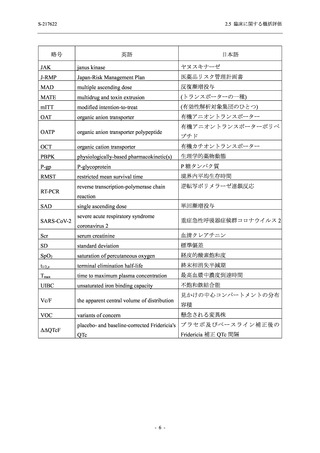
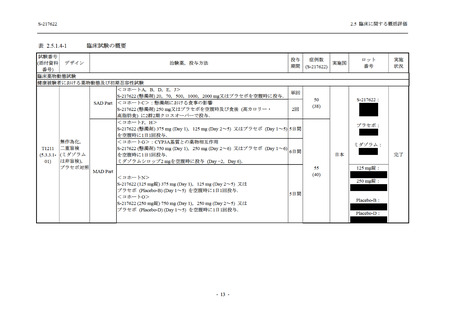
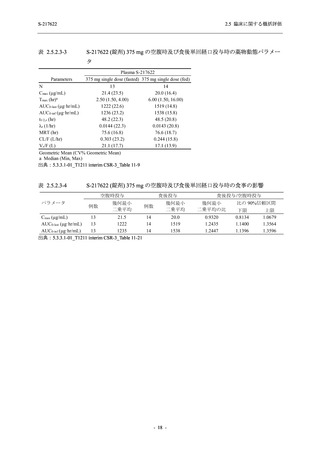
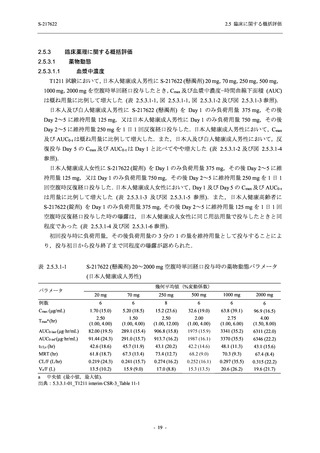
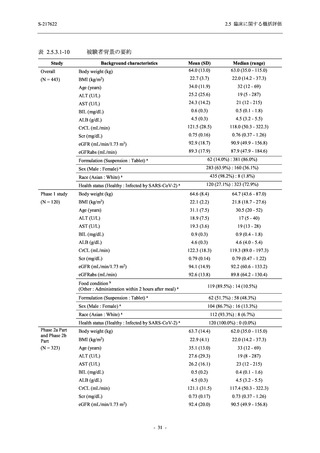
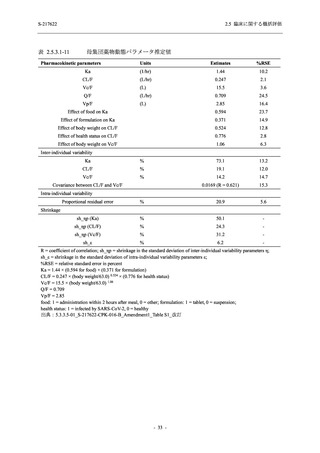
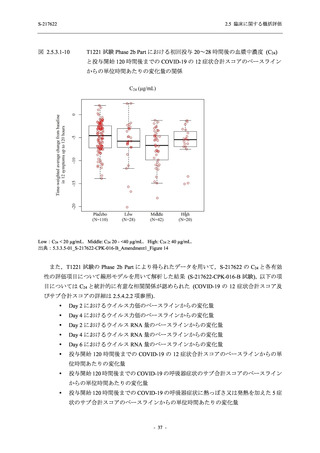
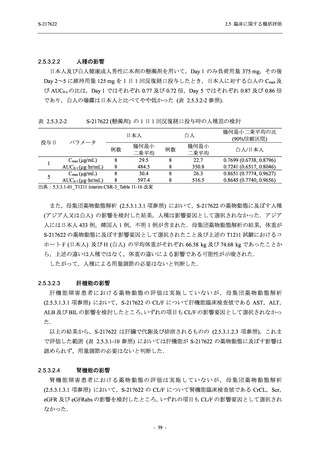
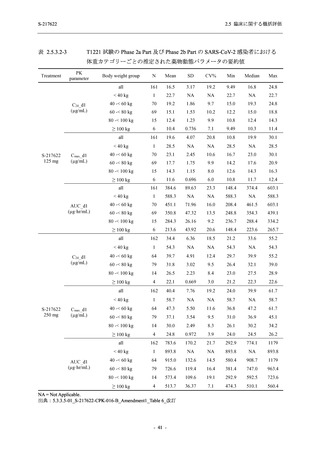
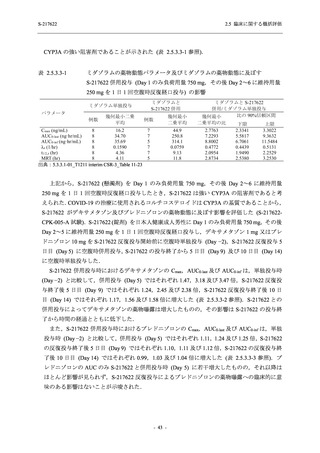
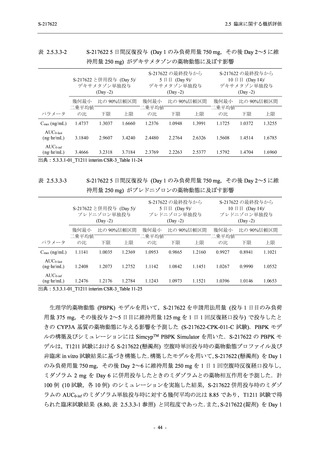
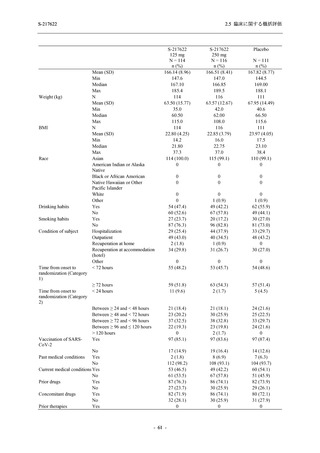
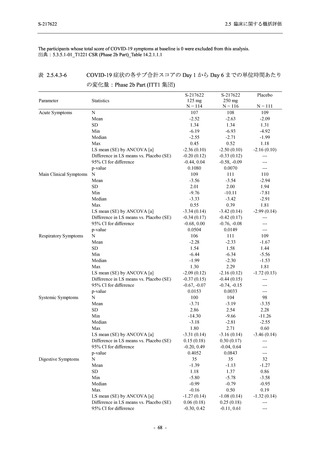
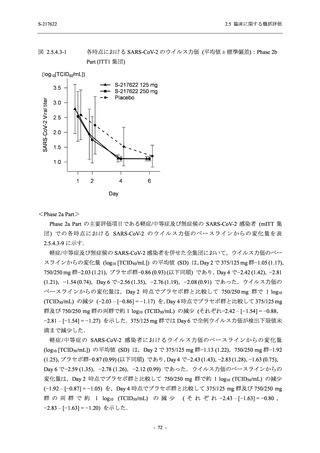
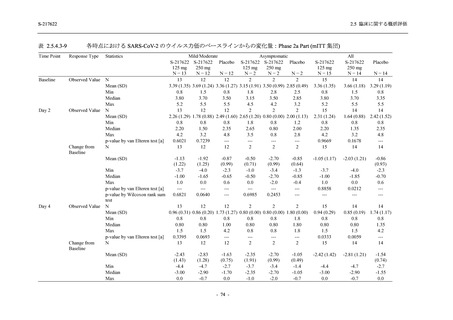
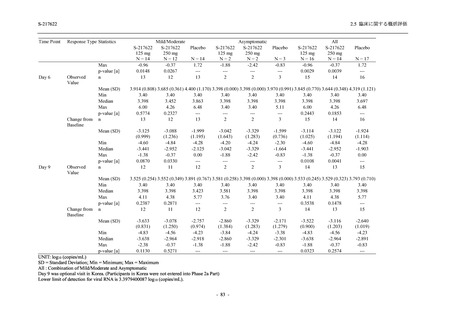
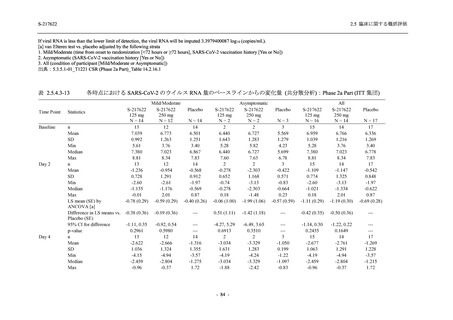
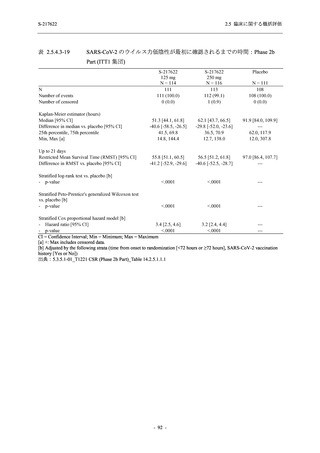
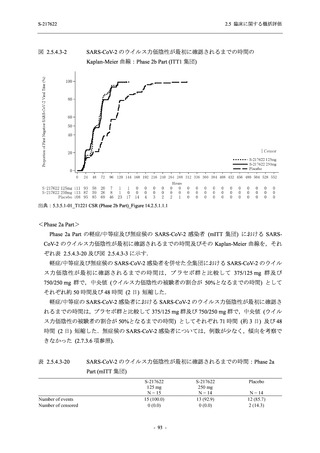
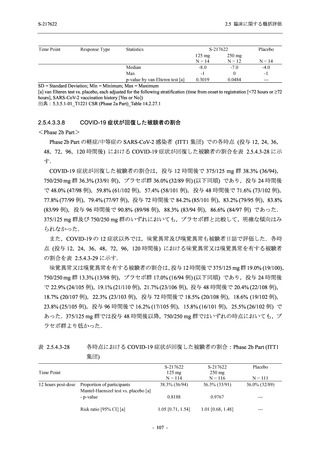
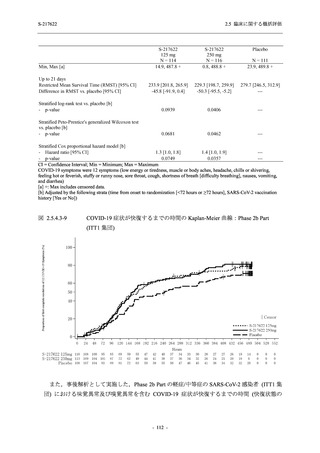
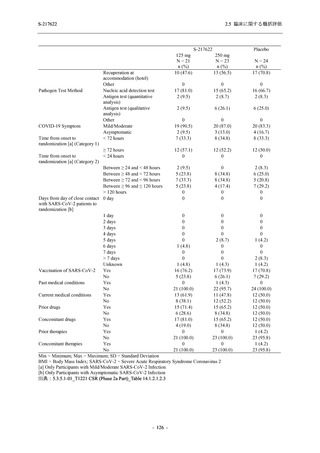
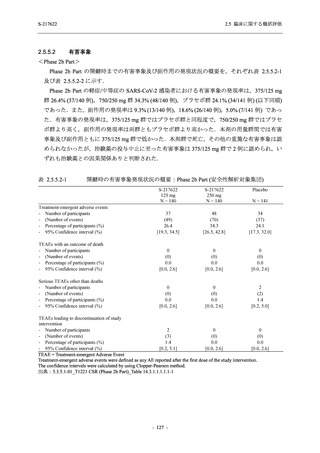
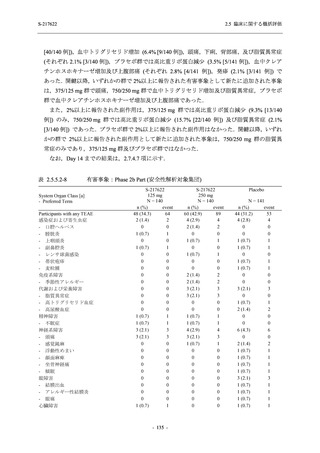
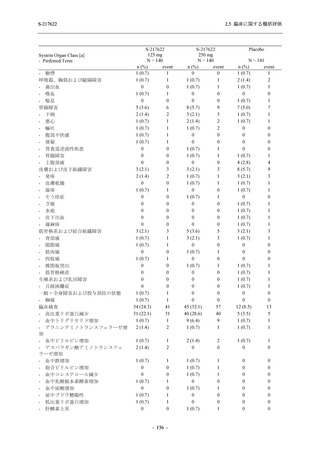
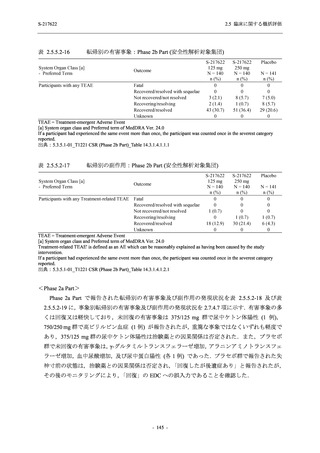
表 2.5.5.2-5
2.5 臨床に関する概括評価
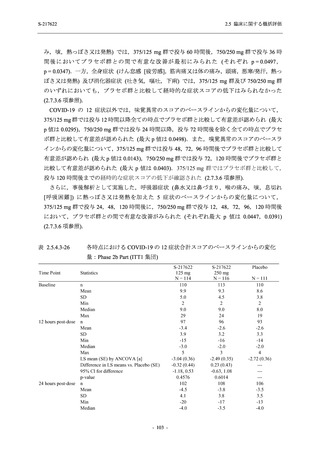
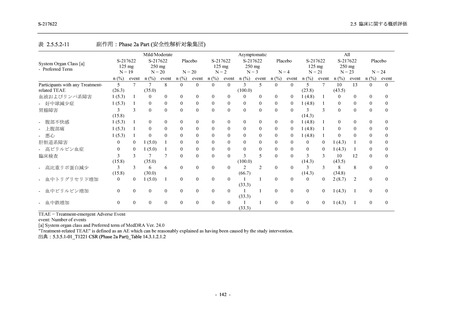
有害事象及び副作用発現状況の概要:Phase 2a Part (安全性解析対象集団)
S-217622
125 mg
N = 19
n (%)
Mild/Moderate
S-217622
Placebo
250 mg
N = 20
N = 20
n (%)
n (%)
S-217622
125 mg
N=2
n (%)
Asymptomatic
S-217622
Placebo
250 mg
N=3
N=4
n (%)
n (%)
S-217622
125 mg
N = 21
n (%)
All
S-217622
250 mg
N = 23
n (%)
Placebo
N = 24
n (%)
Treatment-emergent adverse events
- Number of participants
- (Number of events)
- Percentage of participants (%)
- 95% Confidence interval (%)
10
13
9
1
3
0
11
16
9
(20)
(31)
(17)
(1)
(6)
(0)
(21)
(37)
(17)
52.6
65.0
45.0
50.0
100.0
0.0
52.4
69.6
37.5
[28.9, 75.6] [40.8, 84.6] [23.1, 68.5] [1.3, 98.7] [29.2, 100.0] [0.0, 60.2] [29.8, 74.3] [47.1, 86.8] [18.8, 59.4]
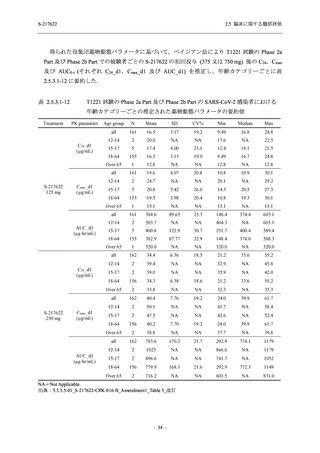
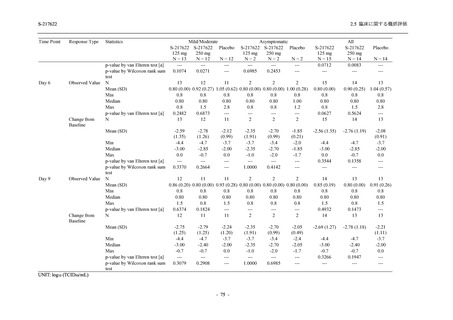
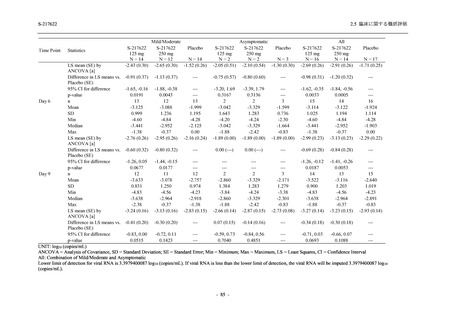
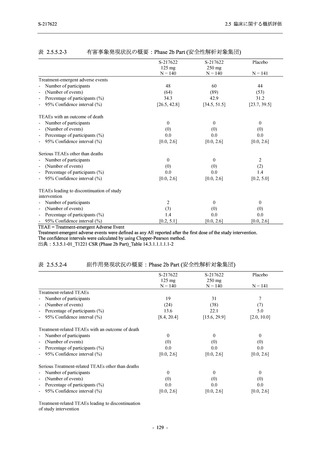
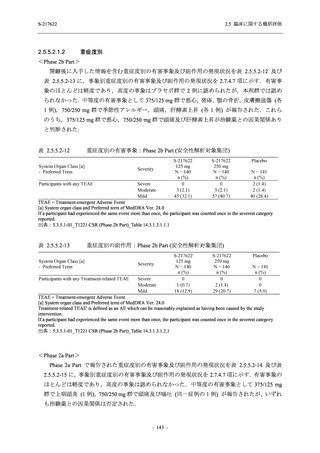
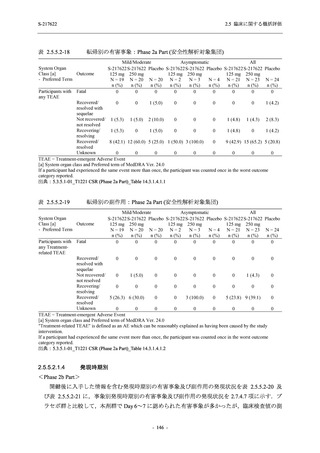
Treatment-related TEAEs
- Number of participants
- (Number of events)
- Percentage of participants (%)
- 95% Confidence interval (%)
5
7
0
(7)
(8)
(0)
26.3
35.0
0.0
[9.1, 51.2] [15.4, 59.2] [0.0, 16.8]
0
3
0
(0)
(5)
(0)
0.0
100.0
0.0
[0.0, 84.2] [29.2, 100.0] [0.0, 60.2]
5
10
0
(7)
(13)
(0)
23.8
43.5
0.0
[8.2, 47.2] [23.2, 65.5] [0.0, 14.2]
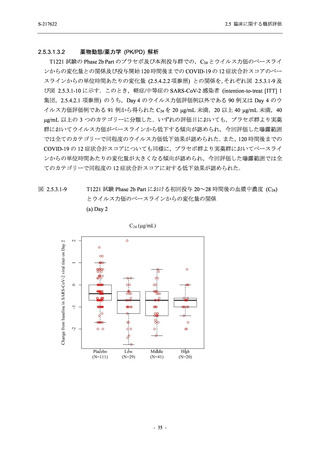
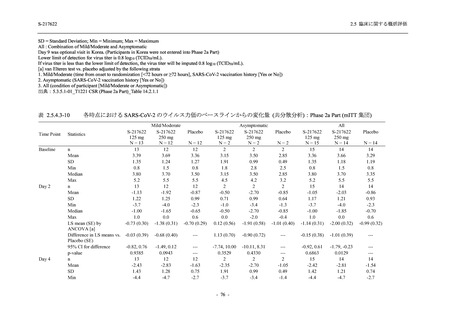
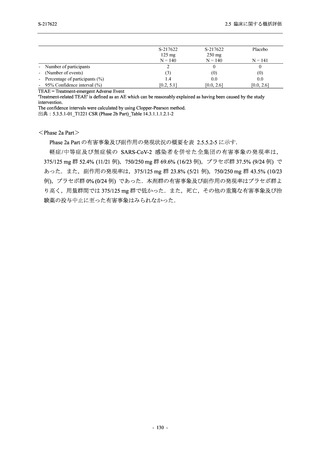
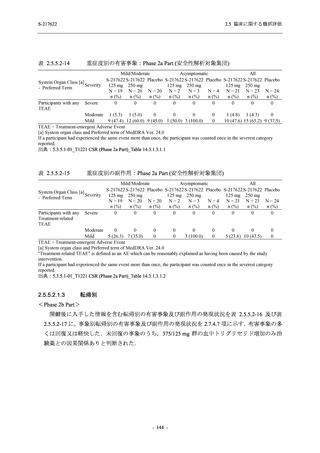
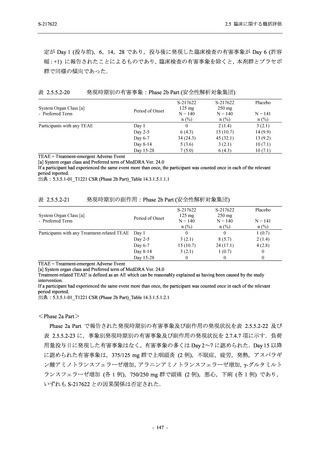
TEAEs with an outcome of death
- Number of participants
- (Number of events)
- Percentage of participants (%)
- 95% Confidence interval (%)
0
(0)
0.0
[0.0, 17.6]
0
(0)
0.0
[0.0, 16.8]
0
(0)
0.0
[0.0, 16.8]
0
(0)
0.0
[0.0, 84.2]
0
(0)
0.0
[0.0, 70.8]
0
(0)
0.0
[0.0, 60.2]
0
(0)
0.0
[0.0, 16.1]
0
(0)
0.0
[0.0, 14.8]
0
(0)
0.0
[0.0, 14.2]
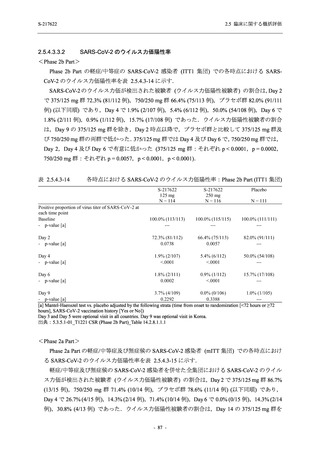
Serious TEAEs other than deaths
- Number of participants
- (Number of events)
- Percentage of participants (%)
- 95% Confidence interval (%)
0
(0)
0.0
[0.0, 17.6]
0
(0)
0.0
[0.0, 16.8]
0
(0)
0.0
[0.0, 16.8]
0
(0)
0.0
[0.0, 84.2]
0
(0)
0.0
[0.0, 70.8]
0
(0)
0.0
[0.0, 60.2]
0
(0)
0.0
[0.0, 16.1]
0
(0)
0.0
[0.0, 14.8]
0
(0)
0.0
[0.0, 14.2]
0
(0)
0.0
[0.0, 60.2]
0
(0)
0.0
[0.0, 16.1]
0
(0)
0.0
[0.0, 14.8]
0
(0)
0.0
[0.0, 14.2]
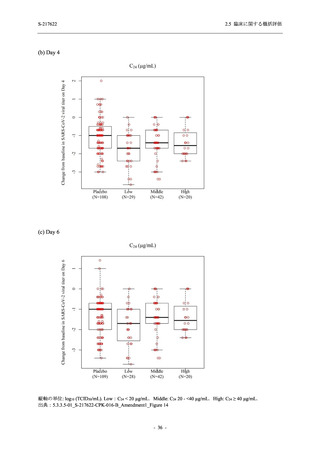
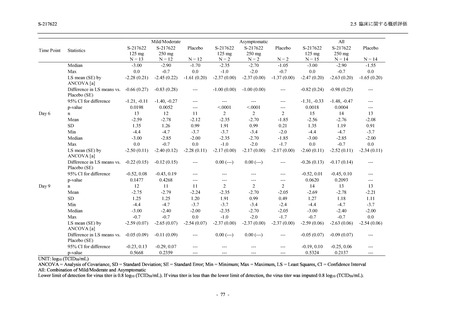
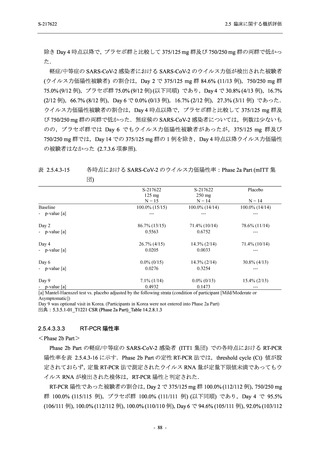
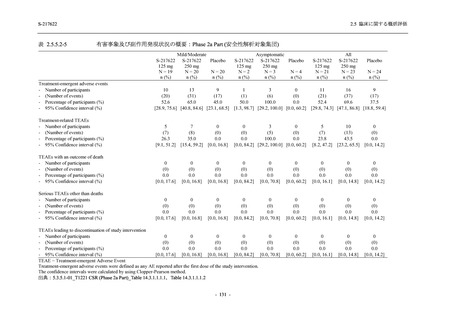
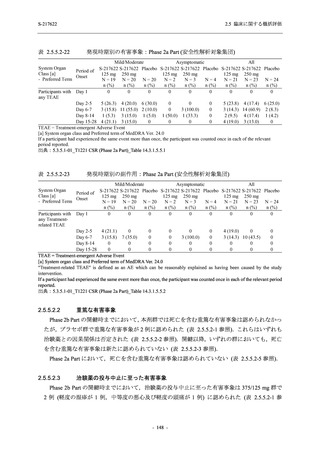
TEAEs leading to discontinuation of study intervention
- Number of participants
0
0
0
0
0
- (Number of events)
(0)
(0)
(0)
(0)
(0)
- Percentage of participants (%)
0.0
0.0
0.0
0.0
0.0
- 95% Confidence interval (%)
[0.0, 17.6] [0.0, 16.8] [0.0, 16.8] [0.0, 84.2] [0.0, 70.8]
TEAE = Treatment-emergent Adverse Event
Treatment-emergent adverse events were defined as any AE reported after the first dose of the study intervention.
The confidence intervals were calculated by using Clopper-Pearson method.
出典:5.3.5.1-01_T1221 CSR (Phase 2a Part)_Table 14.3.1.1.1.1,Table 14.3.1.1.1.2
- 131 -