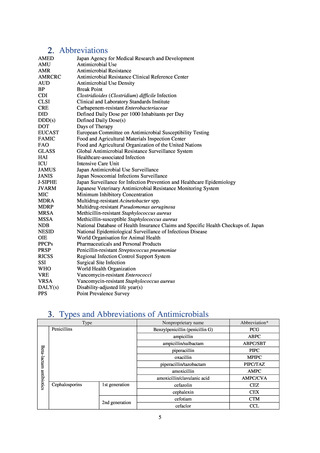
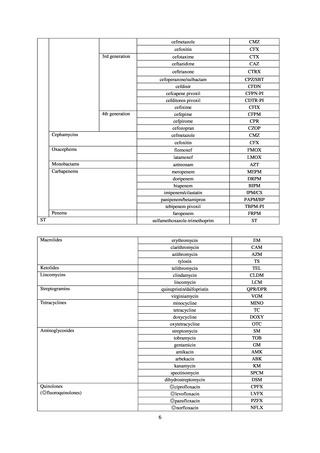
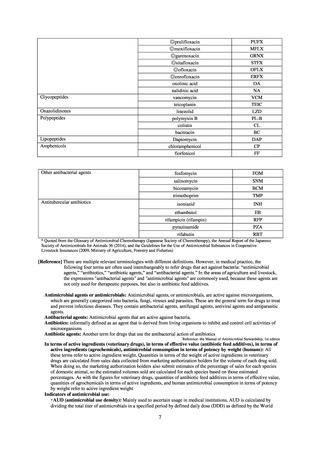
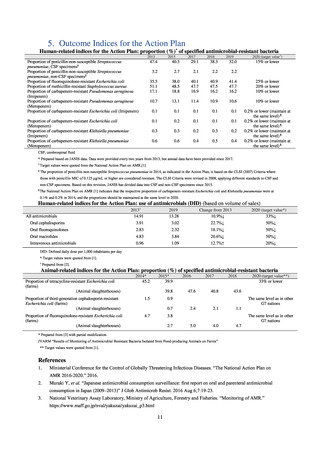
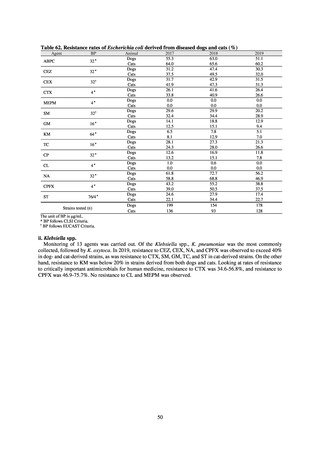
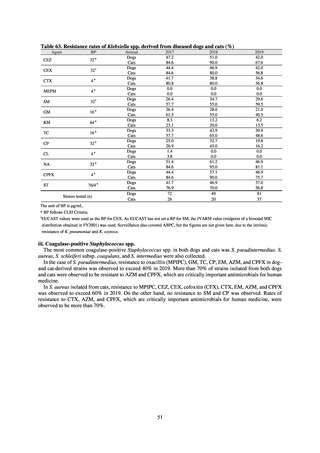
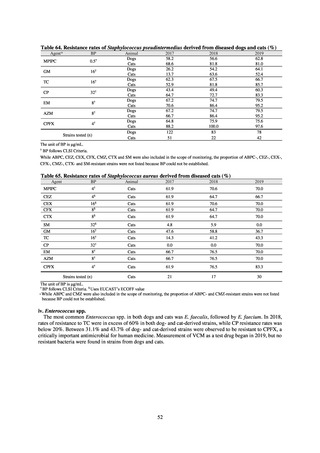
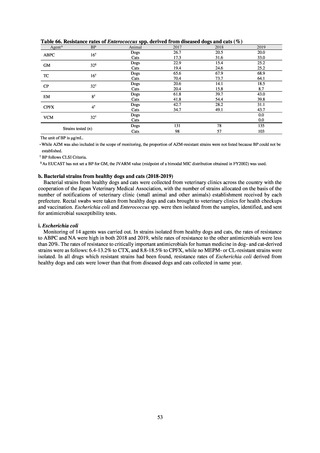
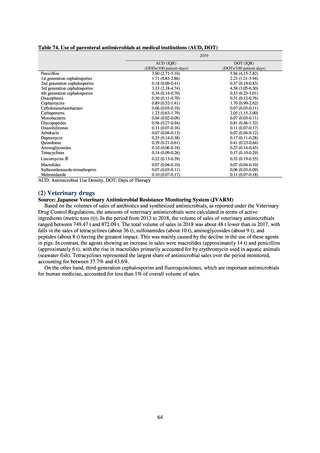
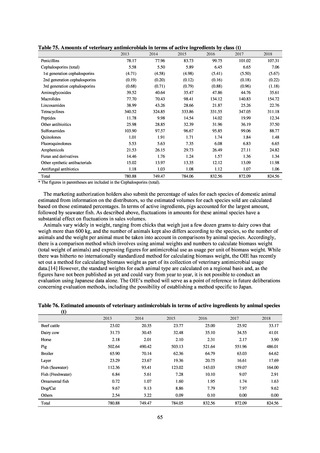
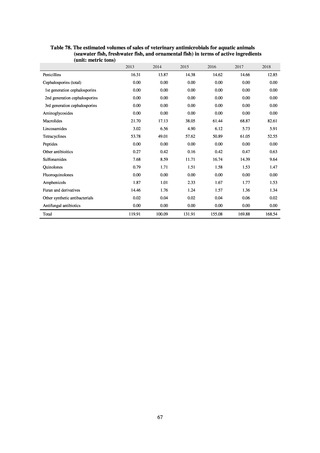
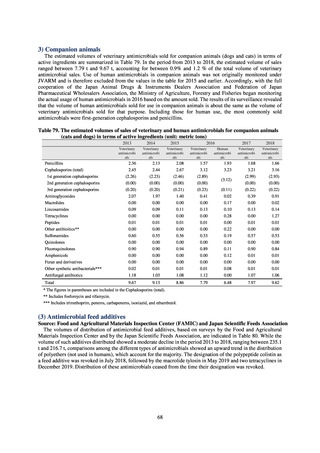
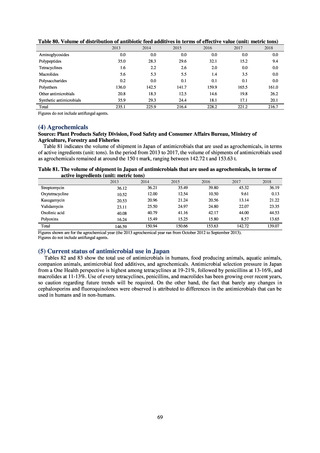
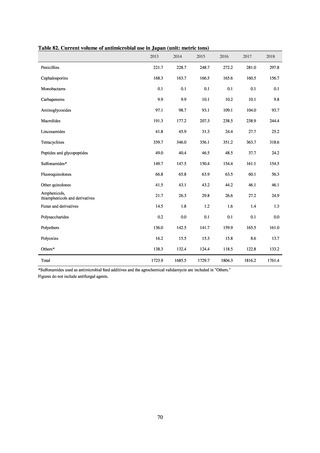
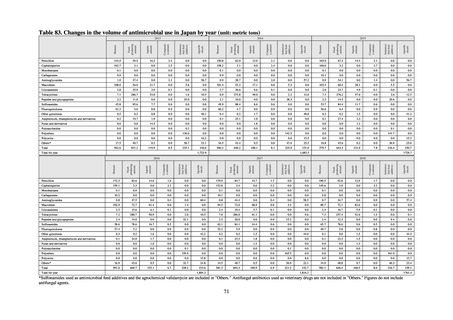
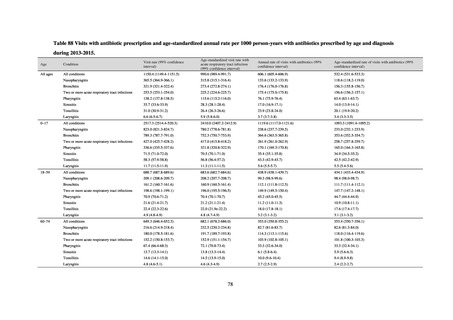
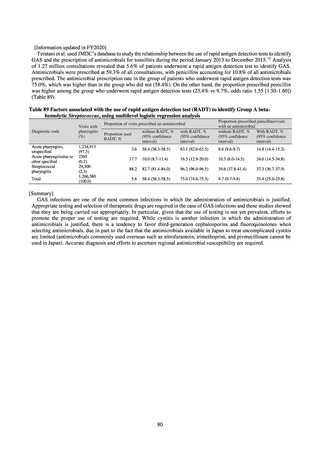
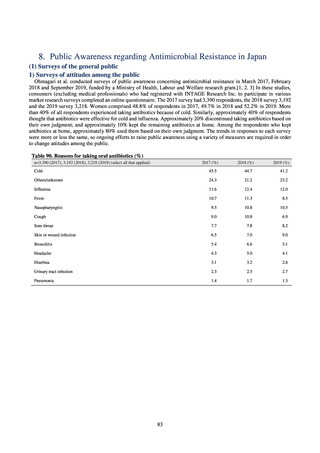
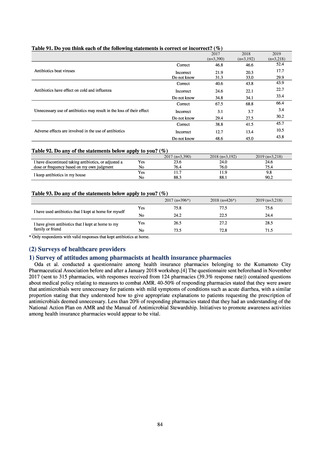
【参考資料3】【英版R4.1.17】Nippon AMR One Health Report (NAOR) 2020 (97 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23261.html |
| 出典情報 | 国際的に脅威となる感染症対策関係閣僚会議 薬剤耐性ワンヘルス動向調査検討会(第9回 1/17)《厚生労働省》 |
ページ画像
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
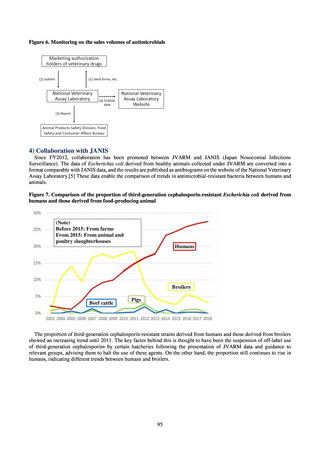
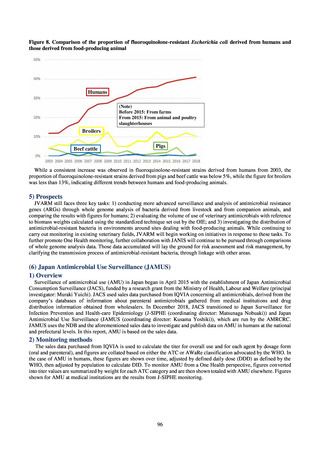
those derived from food-producing animal
Humans
(Note)
Before 2015: From farms
From 2015: From animal and poultry
slaughterhouses
Broilers
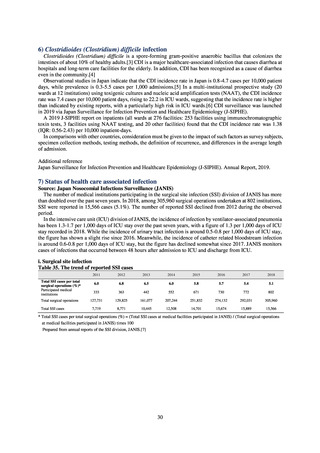
Beef cattle
Pigs
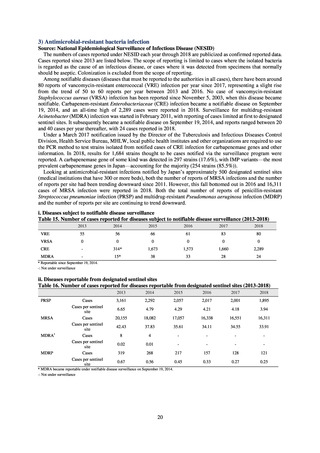
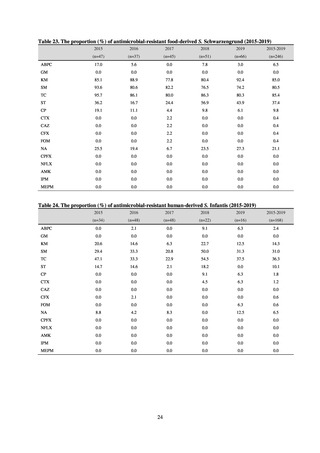
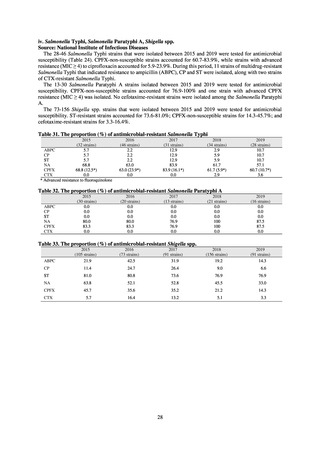
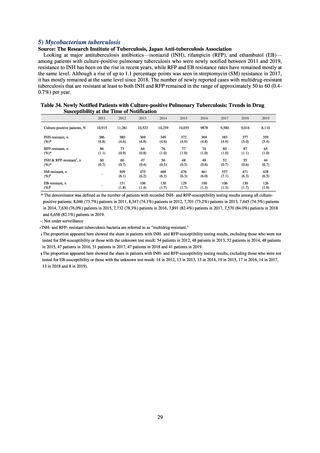
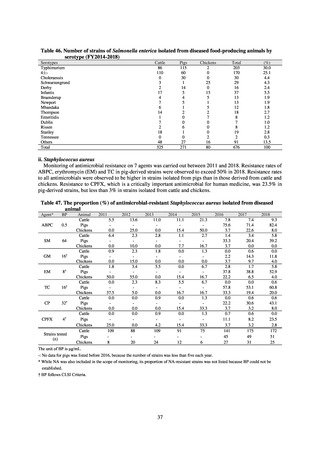
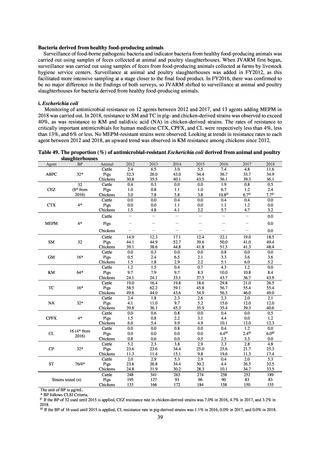
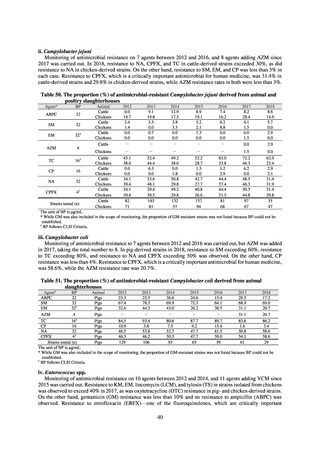
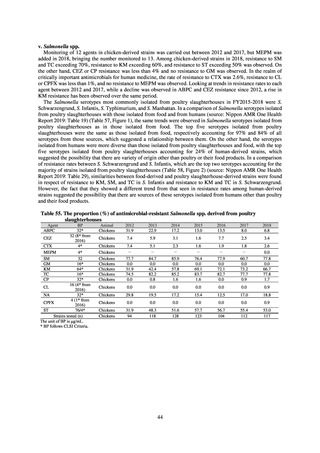
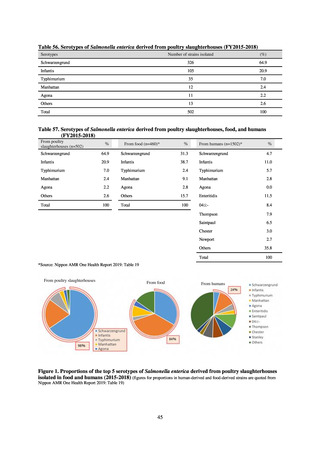
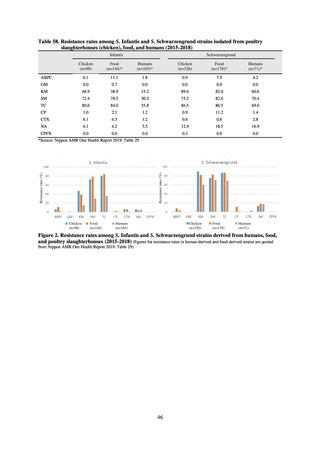
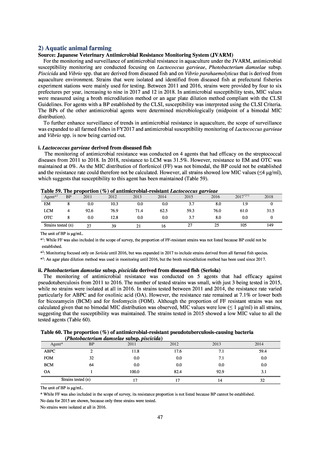
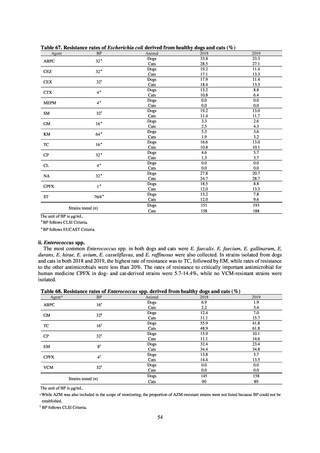
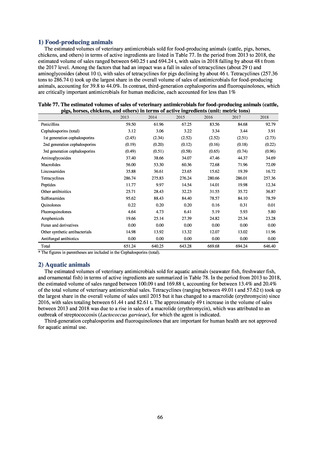
While a consistent increase was observed in fluoroquinolone-resistant strains derived from humans from 2003, the
proportion of fluoroquinolone-resistant strains derived from pigs and beef cattle was below 5%, while the figure for broilers
was less than 13%, indicating different trends between humans and food-producing animals.
5) Prospects
JVARM still faces three key tasks: 1) conducting more advanced surveillance and analysis of antimicrobial resistance
genes (ARGs) through whole genome analysis of bacteria derived from livestock and from companion animals, and
comparing the results with figures for humans; 2) evaluating the volume of use of veterinary antimicrobials with reference
to biomass weights calculated using the standardized technique set out by the OIE; and 3) investigating the distribution of
antimicrobial-resistant bacteria in environments around sites dealing with food-producing animals. While continuing to
carry out monitoring in existing veterinary fields, JVARM will begin working on initiatives in response to these tasks. To
further promote One Health monitoring, further collaboration with JANIS will continue to be pursued through comparisons
of whole genome analysis data. Those data accumulated will lay the ground for risk assessment and risk management, by
clarifying the transmission process of antimicrobial-resistant bacteria, through linkage with other areas.
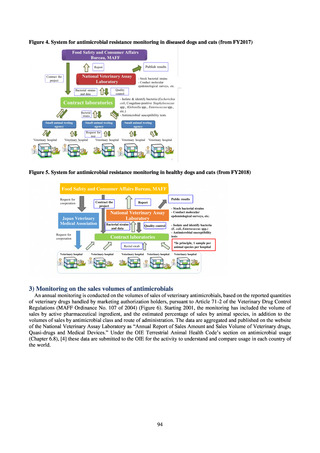
(6) Japan Antimicrobial Use Surveillance (JAMUS)
1) Overview
Surveillance of antimicrobial use (AMU) in Japan began in April 2015 with the establishment of Japan Antimicrobial
Consumption Surveillance (JACS), funded by a research grant from the Ministry of Health, Labour and Welfare (principal
investigator: Muraki Yuichi). JACS used sales data purchased from IQVIA concerning all antimicrobials, derived from the
company’s databases of information about parenteral antimicrobials gathered from medical institutions and drug
distribution information obtained from wholesalers. In December 2018, JACS transitioned to Japan Surveillance for
Infection Prevention and Health‐care Epidemiology (J-SIPHE (coordinating director: Matsunaga Nobuaki)) and Japan
Antimicrobial Use Surveillance (JAMUS (coordinating director: Kusama Yoshiki)), which are run by the AMRCRC.
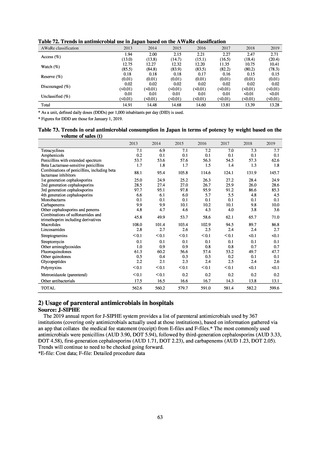
JAMUS uses the NDB and the aforementioned sales data to investigate and publish data on AMU in humans at the national
and prefectural levels. In this report, AMU is based on the sales data.
2) Monitoring methods
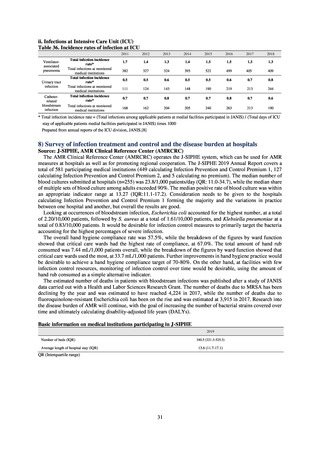
The sales data purchased from IQVIA is used to calculate the titer for overall use and for each agent by dosage form
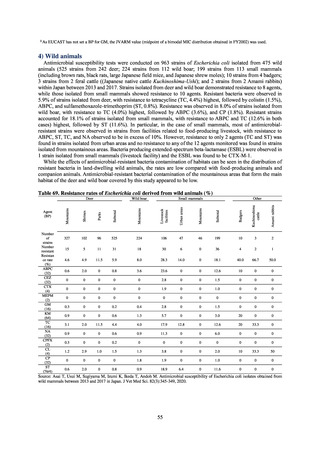
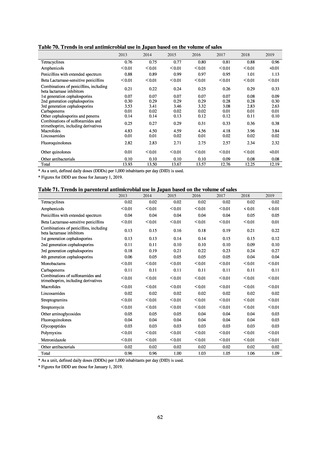
(oral and parenteral), and figures are collated based on either the ATC or AWaRe classification advocated by the WHO. In
the case of AMU in humans, these figures are shown over time, adjusted by defined daily dose (DDD) as defined by the
WHO, then adjusted by population to calculate DID. To monitor AMU from a One Health perspective, figures converted
into titer values are summarized by weight for each ATC category and are then shown totaled with AMU elsewhere. Figures
shown for AMU at medical institutions are the results from J-SIPHE monitoring.
96
関連画像
ページ内で利用されている画像ファイルです。