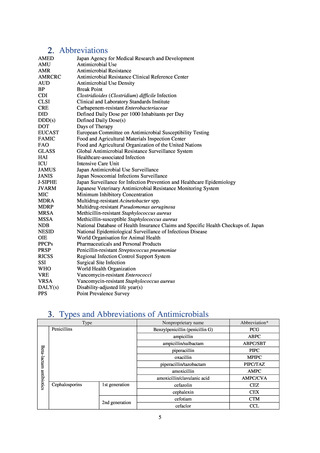
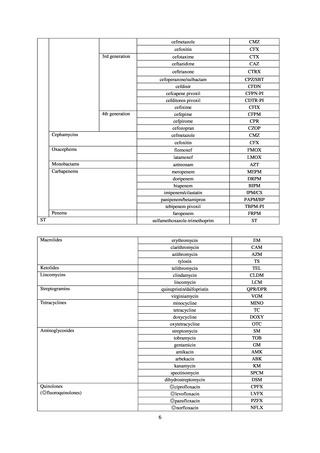
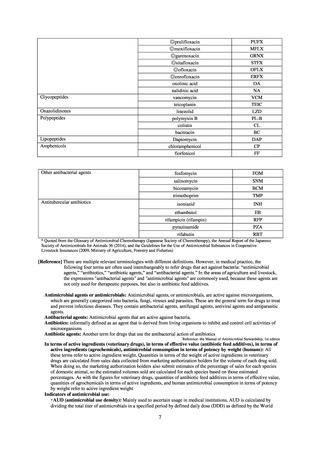
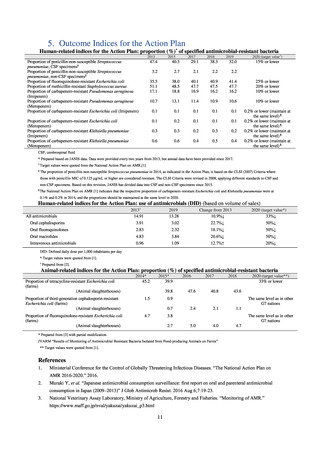
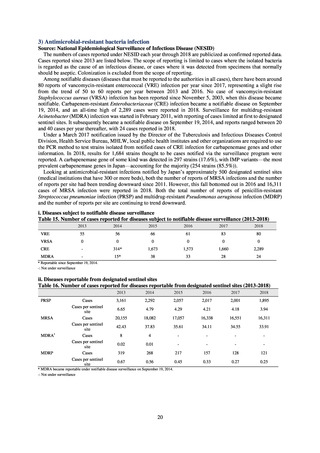
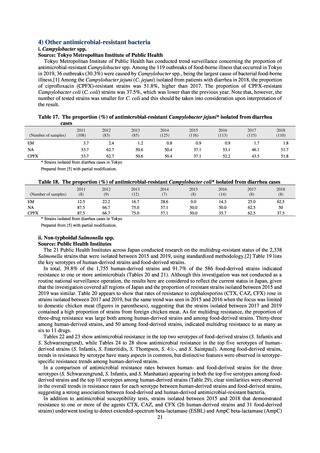
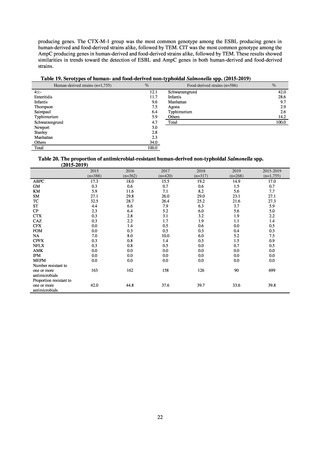
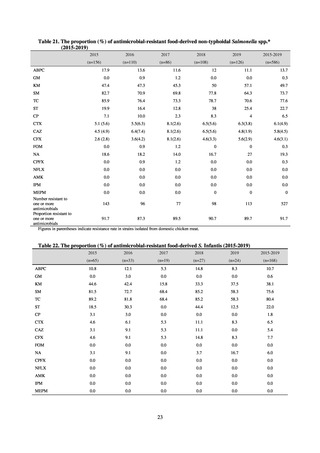
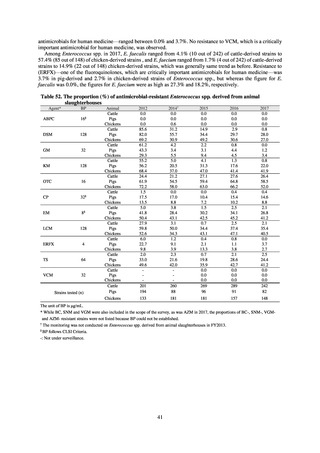
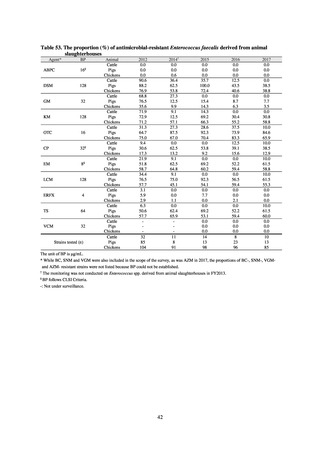
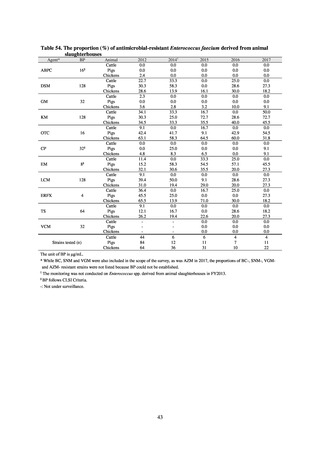
よむ、つかう、まなぶ。
【参考資料3】【英版R4.1.17】Nippon AMR One Health Report (NAOR) 2020 (83 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23261.html |
| 出典情報 | 国際的に脅威となる感染症対策関係閣僚会議 薬剤耐性ワンヘルス動向調査検討会(第9回 1/17)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
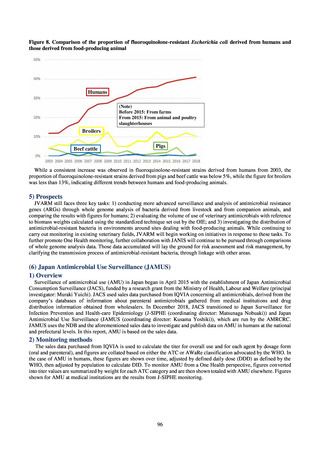
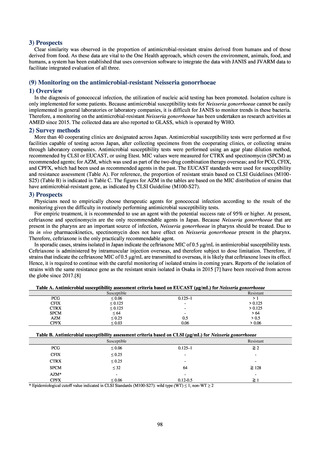
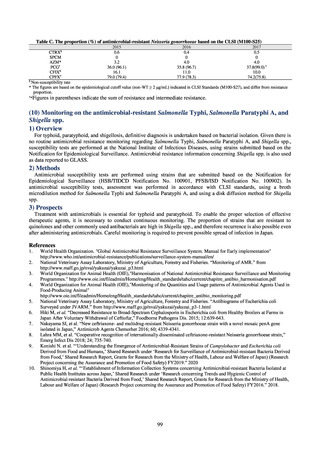
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
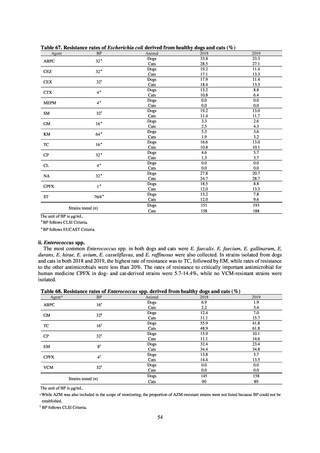
(7) Environment
Pharmaceutical products including antimicrobials, drugs and daily necessities, are collectively referred to as
“Pharmaceuticals and Personal Care Products (PPCPs).” PPCPs may have physiological activity even at low concentration,
causing concerns about effect on aquatic ecosystems.[10] Regarding antimicrobials as a type of PPCPs, several studies
have indicated the measurements of antimicrobial concentrations in the environment (e.g. sewage, treated wastewater,
recycled water, environmental water, and sludge).[11]
In some cases, a part of sewage sludge (biomass) that is generated from sewage treatment is reused as agricultural
fertilizers through anaerobic digestion and composting. The extent to which PPCPs are degraded in the sewage treatment
process or in the sewage sludge digestion process varies by the type of PPCPs. For example, among other antimicrobials,
most sulfonamides are decomposed, while fluoroquinolones, such as ofloxacin and norfloxacin, reside in sludge at high
concentrations without being degraded.[12] The biodegradation process of PPCPs is affected by water temperature. The
removability of PPCPs is affected by treatment conditions in the sewage treatment process, such as hydraulic retention
time, the processing concentration and retention time of activated sludge. To further promote removal, research is in
progress to improve the removability of antimicrobials using membrane bioreactor.[10] Many research activities are also
undertaken both in Japan and overseas to improve efficiency in removing antimicrobials, by introducing ozone and
advanced oxidation process. It is required to identify the current status of discharge and developmental trends in Japan.[11]
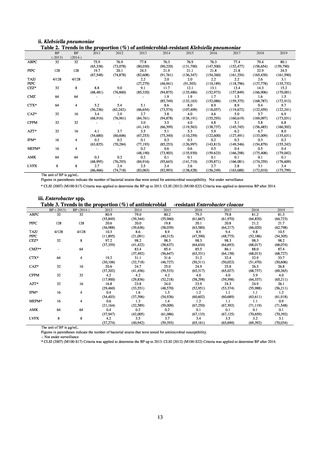
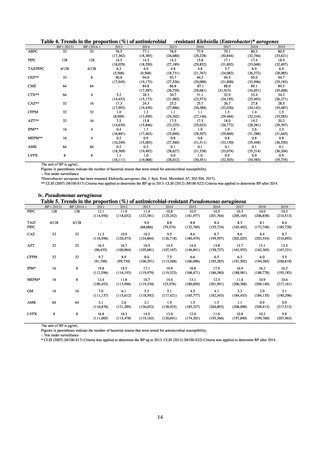
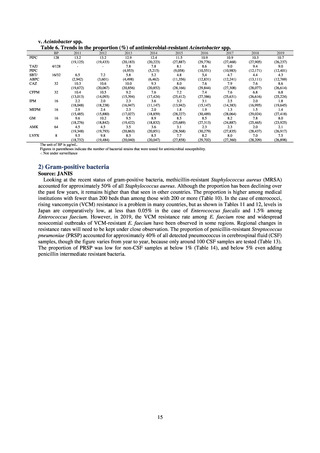
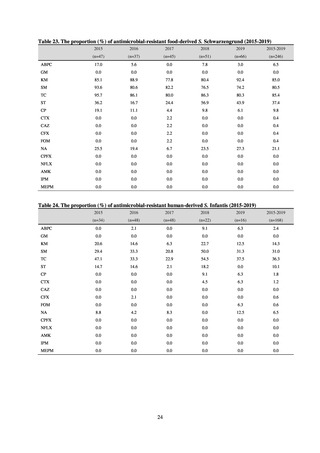
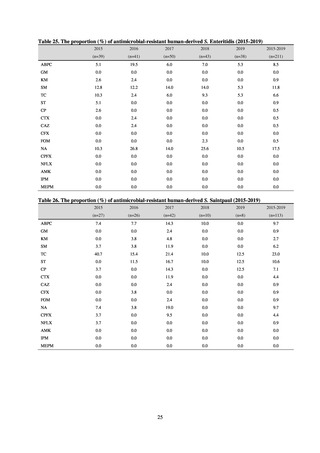
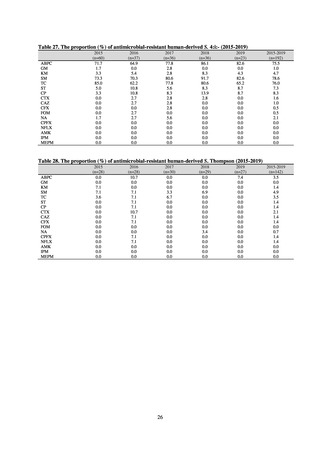
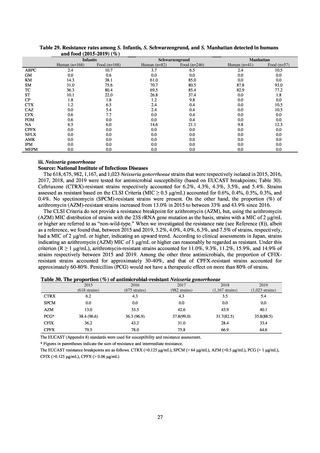
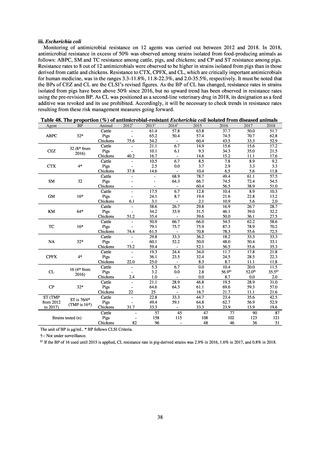
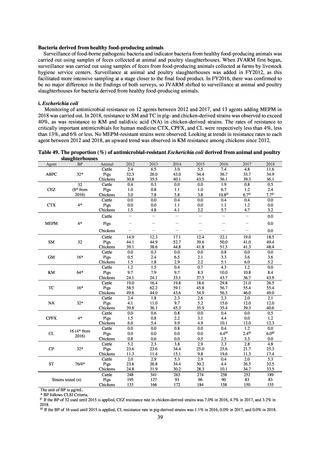
A study that measured the concentrations of antimicrobials detected in Japanese urban rivers, based on influent sewage
at sewage treatment plants, reported that the actual measurements of CPFX and clarithromycin indicated certain similarity
to concentrations expected from the volumes of shipment or sales of these antimicrobials, and pointed out that it may be
possible to predict sewage concentrations of antimicrobials based on their volumes of shipment or sales.[13] The study
reported that, for example, CPFX and clarithromycin were contained in sewage at the respective concentrations of 51 to
442 ng/L and 886 to 1,866 ng/L. However, no research results have been reported that these antimicrobials in the
environment are affecting the health of humans and other living things.
References
1.
Hashimoto H, Matsui H, Sasabuchi Y, Yasunaga H, Kotani K, Nagai R, et al. Antibiotic prescription among outpatients in a
prefecture of Japan, 2012–2013: a retrospective claims database study. BMJ Open [Internet]. 2019 Apr 3
2.
Higashi T, Fukuhara S. Antibiotic prescriptions for upper respiratory tract infection in Japan. Intern Med. 2009;48:1369–75.
3.
Yoshida S, Takeuchi M, Kawakami K. Prescription of antibiotics to pre-school children from 2005 to 2014 in Japan: a
retrospective claims database study. J Public Health (Oxf). 2018;40:397–403.
4.
Teratani Y, Hagiya H, Koyama T, Adachi M, Ohshima A, Zamami Y, et al. Pattern of antibiotic prescriptions for outpatients
with acute respiratory tract infections in Japan, 2013–15: a retrospective observational study. Fam Pract. 2019;36:402–9.
5.
Kimura Y, Fukuda H, Hayakawa K, Ide S, Ota M, et al.. Longitudinal trends of and factors associated with inappropriate
antibiotic prescribing for non-bacterial acute respiratory tract infection in Japan: A retrospective claims database study, 20122017. PLoS One. 2019; 14(10):e0223835.
6.
Tomii K, Matsumura Y, Maeda K, Kobayashi Y, Takano Y, Tasaka Y. Minimal use of antibiotics for acute respiratory tract
infections: validity and patient satisfaction. Intern Med. 2007;46:267–72.
7.
Okubo Y, Michihata N, Morisaki N, Kinoshita N, Miyairi I, Urayama KY, et al. Recent patterns in antibiotic use for children
with group A streptococcal infections in Japan. J Glob Antimicrob Resist. 2018 Jun;13:55–9.
8.
Okubo Y, Miyairi I, Michihata N, Morisaki N, Kinoshita N, Urayama KY, et al. Recent Prescription Patterns for Children With
Acute Infectious Diarrhea. J Pediatr Gastroenterol Nutr. 2019;68:13–6.
9.
Karen E. Jerardi and Elizabeth C. Jackson. Nelson Textbook of Pediatrics, Chapter 553, 2789-2795.e1
10.
Tanaka H, et al. “Contamination of the Aquatic Environment by PPCPs, and Development of Reducing Technology.”
Environmental Technology, Vo. 37, No. 12., 2008.
11.
Park J, et al. “Removal characteristics of PPCPs: comparison between membrane bioreactor and various biological treatment
process.” Chemosphere. 2017; 179: 347e358.
12.
Narumiya M, et al. “Phase distribution and removal of PPCPs during anaerobic sludge digestion” Journal of Hazardous Materials
2013; 260: 305 - 312.
13. Azuma T, et al. “Evaluation of concentrations of pharmaceuticals detected in sewage influents in Japan by using annual shipping
and sales data” Chemosphere. 2015;138 :770 -776.
14.
World Organization for Animal Health (OIE), "Monitoring of the Quantities and Usage patterns of Antimicrobial Agents Used in
Food-Producing Animal"
http://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_antibio_monitoring.pdf
82
Pharmaceutical products including antimicrobials, drugs and daily necessities, are collectively referred to as
“Pharmaceuticals and Personal Care Products (PPCPs).” PPCPs may have physiological activity even at low concentration,
causing concerns about effect on aquatic ecosystems.[10] Regarding antimicrobials as a type of PPCPs, several studies
have indicated the measurements of antimicrobial concentrations in the environment (e.g. sewage, treated wastewater,
recycled water, environmental water, and sludge).[11]
In some cases, a part of sewage sludge (biomass) that is generated from sewage treatment is reused as agricultural
fertilizers through anaerobic digestion and composting. The extent to which PPCPs are degraded in the sewage treatment
process or in the sewage sludge digestion process varies by the type of PPCPs. For example, among other antimicrobials,
most sulfonamides are decomposed, while fluoroquinolones, such as ofloxacin and norfloxacin, reside in sludge at high
concentrations without being degraded.[12] The biodegradation process of PPCPs is affected by water temperature. The
removability of PPCPs is affected by treatment conditions in the sewage treatment process, such as hydraulic retention
time, the processing concentration and retention time of activated sludge. To further promote removal, research is in
progress to improve the removability of antimicrobials using membrane bioreactor.[10] Many research activities are also
undertaken both in Japan and overseas to improve efficiency in removing antimicrobials, by introducing ozone and
advanced oxidation process. It is required to identify the current status of discharge and developmental trends in Japan.[11]
A study that measured the concentrations of antimicrobials detected in Japanese urban rivers, based on influent sewage
at sewage treatment plants, reported that the actual measurements of CPFX and clarithromycin indicated certain similarity
to concentrations expected from the volumes of shipment or sales of these antimicrobials, and pointed out that it may be
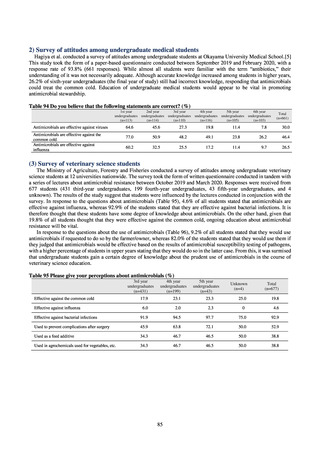
possible to predict sewage concentrations of antimicrobials based on their volumes of shipment or sales.[13] The study
reported that, for example, CPFX and clarithromycin were contained in sewage at the respective concentrations of 51 to
442 ng/L and 886 to 1,866 ng/L. However, no research results have been reported that these antimicrobials in the
environment are affecting the health of humans and other living things.
References
1.
Hashimoto H, Matsui H, Sasabuchi Y, Yasunaga H, Kotani K, Nagai R, et al. Antibiotic prescription among outpatients in a
prefecture of Japan, 2012–2013: a retrospective claims database study. BMJ Open [Internet]. 2019 Apr 3
2.
Higashi T, Fukuhara S. Antibiotic prescriptions for upper respiratory tract infection in Japan. Intern Med. 2009;48:1369–75.
3.
Yoshida S, Takeuchi M, Kawakami K. Prescription of antibiotics to pre-school children from 2005 to 2014 in Japan: a
retrospective claims database study. J Public Health (Oxf). 2018;40:397–403.
4.
Teratani Y, Hagiya H, Koyama T, Adachi M, Ohshima A, Zamami Y, et al. Pattern of antibiotic prescriptions for outpatients
with acute respiratory tract infections in Japan, 2013–15: a retrospective observational study. Fam Pract. 2019;36:402–9.
5.
Kimura Y, Fukuda H, Hayakawa K, Ide S, Ota M, et al.. Longitudinal trends of and factors associated with inappropriate
antibiotic prescribing for non-bacterial acute respiratory tract infection in Japan: A retrospective claims database study, 20122017. PLoS One. 2019; 14(10):e0223835.
6.
Tomii K, Matsumura Y, Maeda K, Kobayashi Y, Takano Y, Tasaka Y. Minimal use of antibiotics for acute respiratory tract
infections: validity and patient satisfaction. Intern Med. 2007;46:267–72.
7.
Okubo Y, Michihata N, Morisaki N, Kinoshita N, Miyairi I, Urayama KY, et al. Recent patterns in antibiotic use for children
with group A streptococcal infections in Japan. J Glob Antimicrob Resist. 2018 Jun;13:55–9.
8.
Okubo Y, Miyairi I, Michihata N, Morisaki N, Kinoshita N, Urayama KY, et al. Recent Prescription Patterns for Children With
Acute Infectious Diarrhea. J Pediatr Gastroenterol Nutr. 2019;68:13–6.
9.
Karen E. Jerardi and Elizabeth C. Jackson. Nelson Textbook of Pediatrics, Chapter 553, 2789-2795.e1
10.
Tanaka H, et al. “Contamination of the Aquatic Environment by PPCPs, and Development of Reducing Technology.”
Environmental Technology, Vo. 37, No. 12., 2008.
11.
Park J, et al. “Removal characteristics of PPCPs: comparison between membrane bioreactor and various biological treatment
process.” Chemosphere. 2017; 179: 347e358.
12.
Narumiya M, et al. “Phase distribution and removal of PPCPs during anaerobic sludge digestion” Journal of Hazardous Materials
2013; 260: 305 - 312.
13. Azuma T, et al. “Evaluation of concentrations of pharmaceuticals detected in sewage influents in Japan by using annual shipping
and sales data” Chemosphere. 2015;138 :770 -776.
14.
World Organization for Animal Health (OIE), "Monitoring of the Quantities and Usage patterns of Antimicrobial Agents Used in
Food-Producing Animal"
http://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_antibio_monitoring.pdf
82