よむ、つかう、まなぶ。
【参考資料3】【英版R4.1.17】Nippon AMR One Health Report (NAOR) 2020 (90 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23261.html |
| 出典情報 | 国際的に脅威となる感染症対策関係閣僚会議 薬剤耐性ワンヘルス動向調査検討会(第9回 1/17)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
(2) National Epidemiological Surveillance of Infectious Disease (NESID)
1) Overview
The NESID program collects and publishes domestic information regarding infectious diseases, and monitors the
occurrence of and trends in infectious diseases, based on reports from physicians and veterinarians. At present, the NESID
program is conducted in accordance with the Act on the Prevention of Infectious Diseases and Medical Care for Patients
with Infectious Diseases (hereinafter referred to as "Infectious Diseases Control Law"), which took effect in April 1999.
The goal of NESID is to accurately identify and analyze information regarding the occurrence of infectious diseases and
to rapidly provide and publish the results to the general public and healthcare practitioners, thereby promoting measures
for the effective and adequate prevention, diagnosis and treatment of infectious diseases, and preventing the occurrence
and spread of various infectious diseases, while verifying the detection status and characteristics of circulating pathogens,
and facilitating appropriate infection control measures, through the collection and analysis of pathogen information.
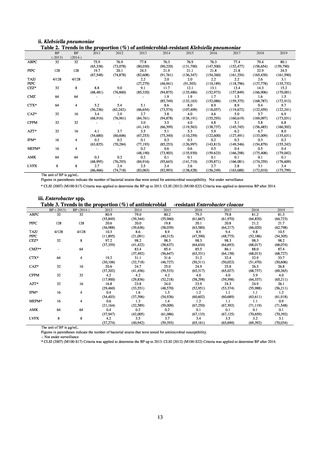
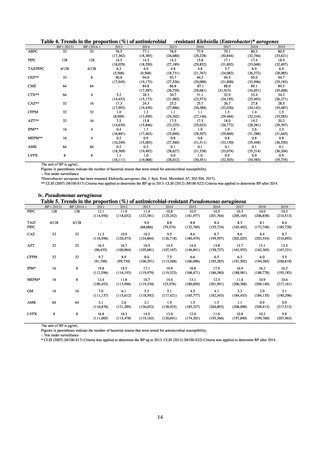
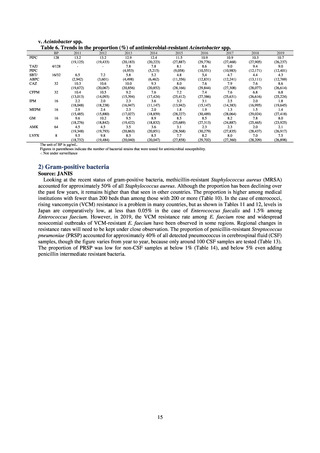
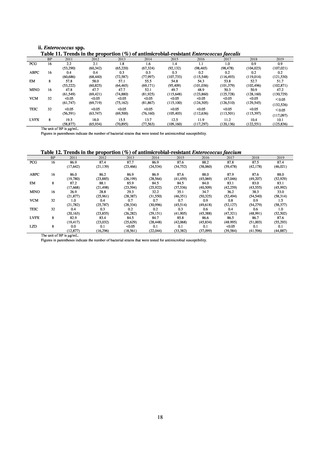
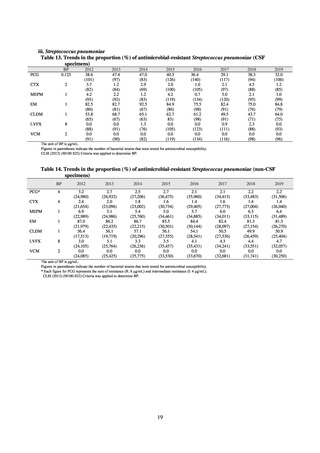
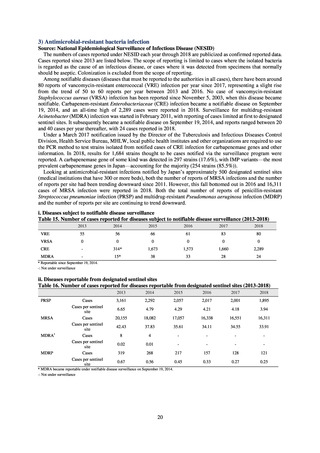
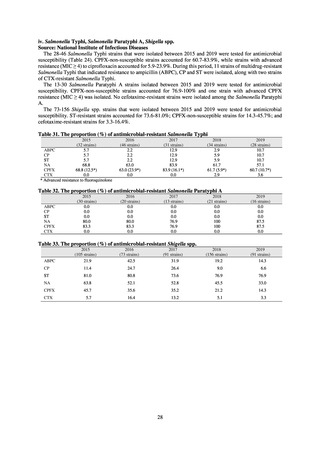
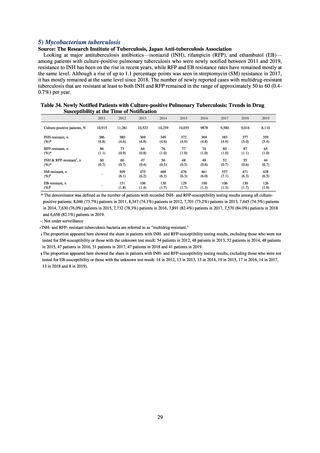
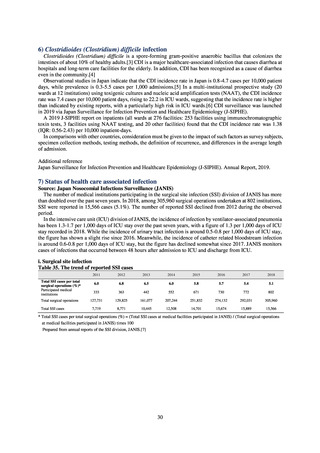
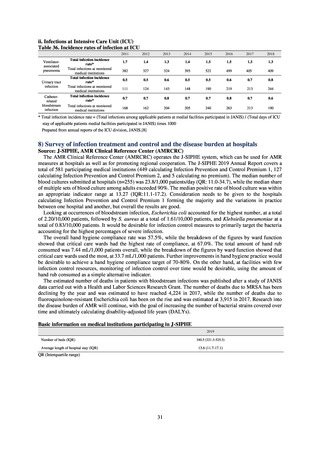
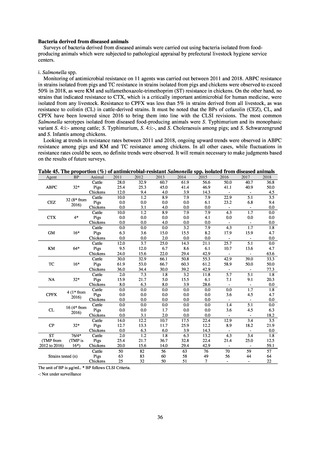
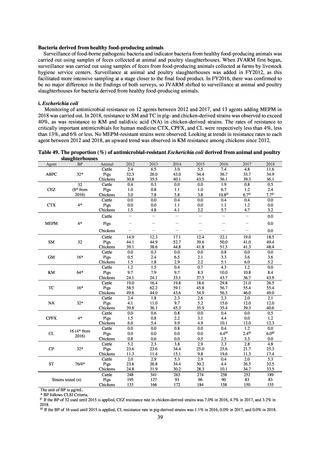
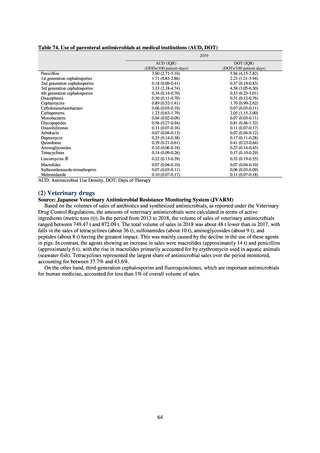
As of July 2019, the following seven antimicrobial-resistant bacteria infections are designated as reportable under
NESID, which are all classified as Category V Infectious Diseases. The four diseases that are subject to notifiable disease
surveillance, which requires reporting by all physicians, are vancomycin-resistant enterococcal infection (VRE, designated
in April 1999), vancomycin-resistant Staphylococcus aureus infection (VRSA, designated in November 2003),
carbapenem-resistant Enterobacteriaceae infection (CRE, designated in September 2014), and multidrug-resistant
Acinetobacter infection (MDRA, designated as a disease reportable from designated sentinel sites in February 2011, and
changed to a disease reportable under notifiable disease surveillance in September 2014). The three diseases that are
reportable from approximately 500 designated sentinel sites (medical institutions that have 300 or more beds, with internal
medicine and surgery departments) across Japan are penicillin-resistant Streptococcus pneumoniae infection (PRSP,
designated in April 1999), methicillin-resistant Staphylococcus aureus infection (MRSA, designated in April 1999), and
multidrug-resistant Pseudomonas aeruginosa infection (MDRP, designated in April 1999).
2) Reporting criteria
A physician who has diagnosed a reportable disease listed above (the manager of a designated notification facility in the
case of a disease subject to sentinel surveillance) should report to a Public Health Center using a designated reporting form.
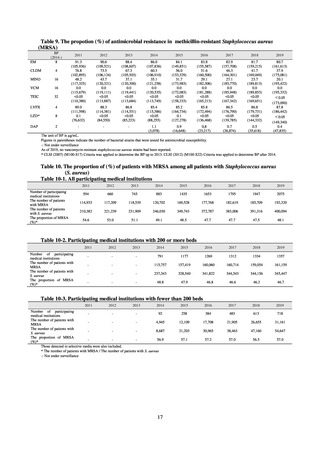
The scope of reporting includes cases where bacteria that satisfy the laboratory findings specified in Table A are detected,
and the isolated bacteria are regarded as the cause of the relevant infectious disease, or cases where it was detected from
specimens that normally should be aseptic. Carriers are excluded from the scope of reporting.
89
1) Overview
The NESID program collects and publishes domestic information regarding infectious diseases, and monitors the
occurrence of and trends in infectious diseases, based on reports from physicians and veterinarians. At present, the NESID
program is conducted in accordance with the Act on the Prevention of Infectious Diseases and Medical Care for Patients
with Infectious Diseases (hereinafter referred to as "Infectious Diseases Control Law"), which took effect in April 1999.
The goal of NESID is to accurately identify and analyze information regarding the occurrence of infectious diseases and
to rapidly provide and publish the results to the general public and healthcare practitioners, thereby promoting measures
for the effective and adequate prevention, diagnosis and treatment of infectious diseases, and preventing the occurrence
and spread of various infectious diseases, while verifying the detection status and characteristics of circulating pathogens,
and facilitating appropriate infection control measures, through the collection and analysis of pathogen information.
As of July 2019, the following seven antimicrobial-resistant bacteria infections are designated as reportable under
NESID, which are all classified as Category V Infectious Diseases. The four diseases that are subject to notifiable disease
surveillance, which requires reporting by all physicians, are vancomycin-resistant enterococcal infection (VRE, designated
in April 1999), vancomycin-resistant Staphylococcus aureus infection (VRSA, designated in November 2003),
carbapenem-resistant Enterobacteriaceae infection (CRE, designated in September 2014), and multidrug-resistant
Acinetobacter infection (MDRA, designated as a disease reportable from designated sentinel sites in February 2011, and
changed to a disease reportable under notifiable disease surveillance in September 2014). The three diseases that are
reportable from approximately 500 designated sentinel sites (medical institutions that have 300 or more beds, with internal
medicine and surgery departments) across Japan are penicillin-resistant Streptococcus pneumoniae infection (PRSP,
designated in April 1999), methicillin-resistant Staphylococcus aureus infection (MRSA, designated in April 1999), and
multidrug-resistant Pseudomonas aeruginosa infection (MDRP, designated in April 1999).
2) Reporting criteria
A physician who has diagnosed a reportable disease listed above (the manager of a designated notification facility in the
case of a disease subject to sentinel surveillance) should report to a Public Health Center using a designated reporting form.
The scope of reporting includes cases where bacteria that satisfy the laboratory findings specified in Table A are detected,
and the isolated bacteria are regarded as the cause of the relevant infectious disease, or cases where it was detected from
specimens that normally should be aseptic. Carriers are excluded from the scope of reporting.
89