よむ、つかう、まなぶ。
【参考資料3】【英版R4.1.17】Nippon AMR One Health Report (NAOR) 2020 (9 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23261.html |
| 出典情報 | 国際的に脅威となる感染症対策関係閣僚会議 薬剤耐性ワンヘルス動向調査検討会(第9回 1/17)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
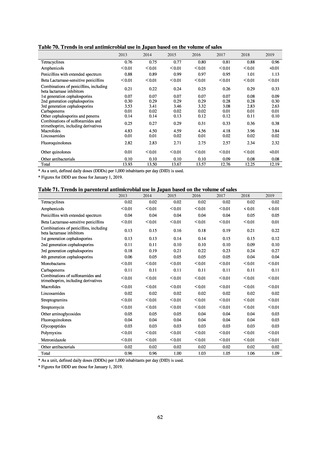
Health Organization (WHO), and correcting the result with the total patient-days. The units used for AUD include
DDDs per 100 bed-days and DDDs per 1,000 patient-days.
・DID (DDDs/1,000 inhabitants/day): Antimicrobial use in a region or country is expressed as DID, which is
calculated by dividing the total titer by DDD and correcting the denominator with the number of inhabitants of the
region per day.
・DOT (day of therapy): DOT is calculated by correcting the total days of therapy (DOTs) using antimicrobials in a
specified period with the total patient-days. The units used for DOT include DOTs per 100 bed-days and DOTs per
1,000 patient-days.
Executive Summary
Background:
Japan’s “National Action Plan on AMR 2016-2020” positions efforts to ascertain the current status of
antimicrobial-resistant bacteria and national antimicrobial use in the areas of human health, animals, food and the
environment and trends therein as an important strategy for both evaluating current policy and examining future
policy. For global monitoring and reporting, WHO has launched the Global Antimicrobial Resistance Surveillance
System (GLASS) for the gathering and sharing of trends in resistant bacteria worldwide. Japan contributes to
GLASS by providing our national data. In addition, Japan also submits data as part of our assistance with an
initiative by the World Organisation for Animal Health (OIE), which uses standardized methods for monitoring
the volume of antimicrobial use in animals. Accordingly, it is crucial for Japan to update both domestic and
overseas stakeholders about the current status and progress of our AMR policy, in order both to reaffirm Japan’s
position in the global community and to accelerate and advance AMR policy internationally.
Method:
The AMR One Health Surveillance Committee, comprised of experts on AMR in the areas of human health,
animals, food and the environment, discussed current surveillance/monitoring systems and reviewed published
research on AMR and antimicrobial use. Data on the proportion of antimicrobial resistance among major
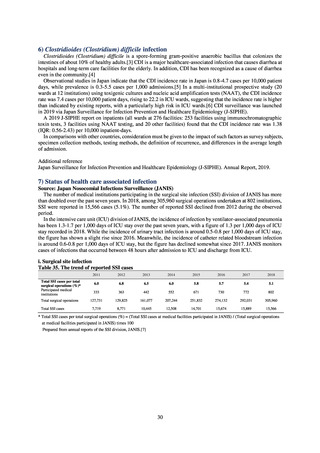
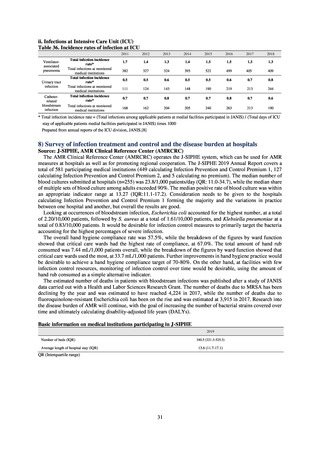
pathogens in the human medical setting were derived from the Japan Nosocomial Infections Surveillance (JANIS)
program organized by the Ministry of Health, Labour and Welfare of Japan. Data on the proportion of
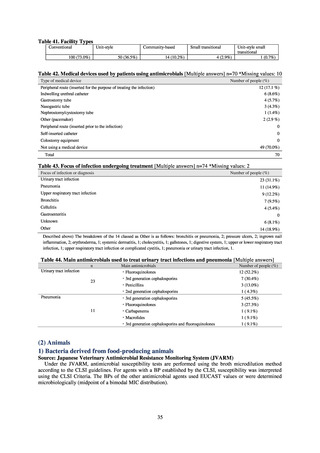
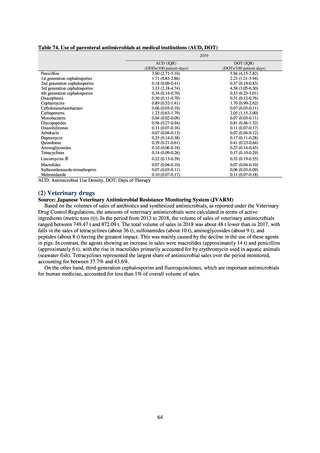
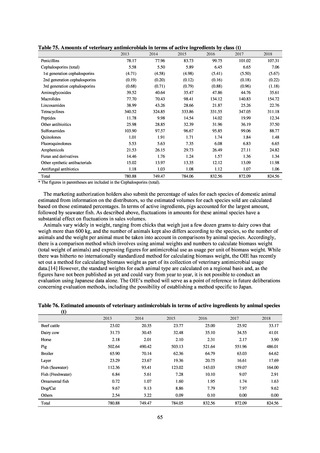
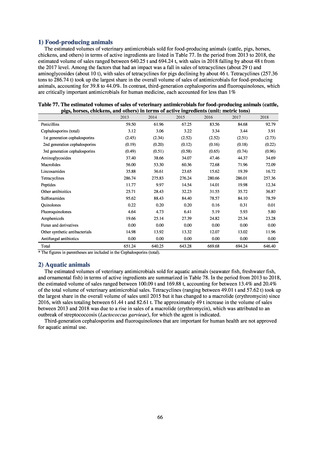
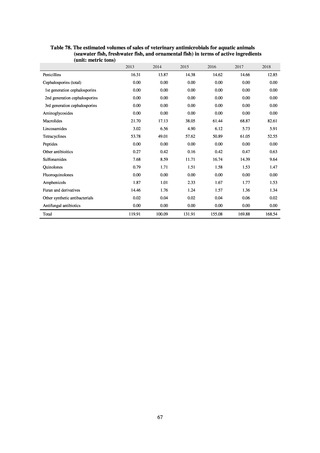
antimicrobial resistance among animals and related antimicrobial sales were derived from the Japanese Veterinary
Antimicrobial Resistance Monitoring System (JVARM) implemented by the Ministry of Agriculture, Forestry and
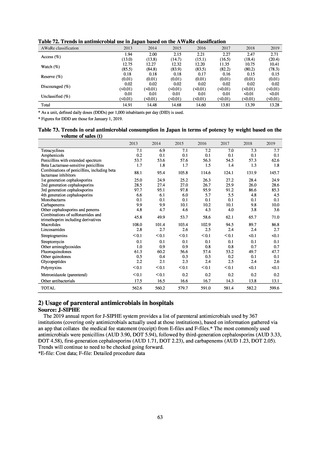
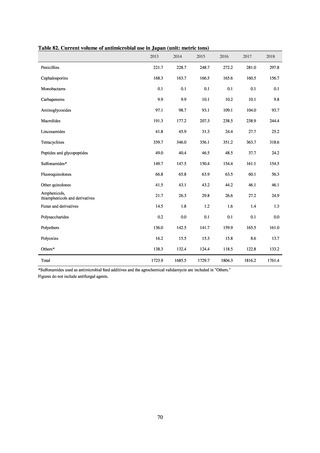
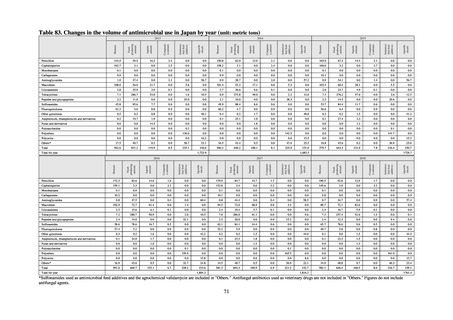
Fisheries of Japan (MAFF). We obtained data on sales and consumption of antimicrobials for human use from
IQVIA Solutions Japan K.K., the National Database of Health Insurance Claims and Specific Health Checkups of
Japan (NDB), and Japan Surveillance for Infection Prevention and Health‐care Epidemiology (J-SIPHE). Data on
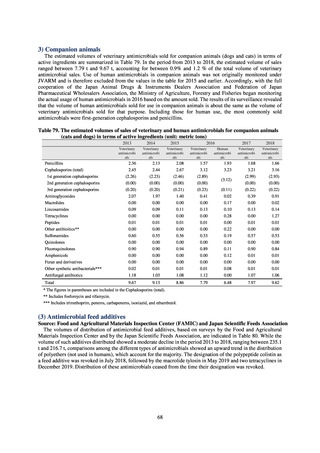
the distribution of antimicrobial feed additives were provided by the Food and Agricultural Materials Inspection
Center (FAMIC) and the Japan Scientific Feeds Associations (JSFA). Data on the volume of domestic shipments
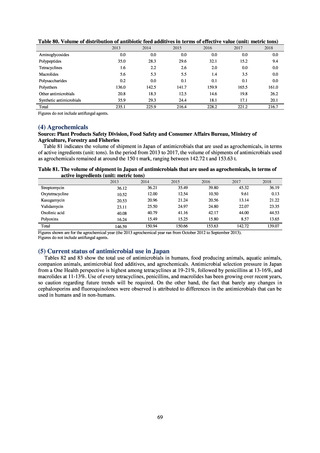
of antimicrobials used as agricultural chemicals was from MAFF.
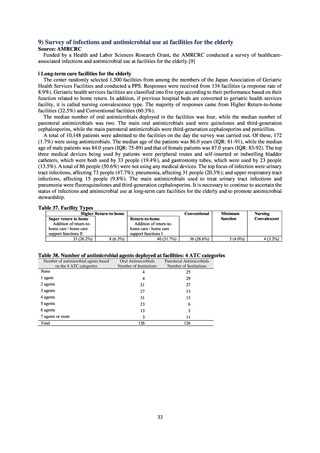
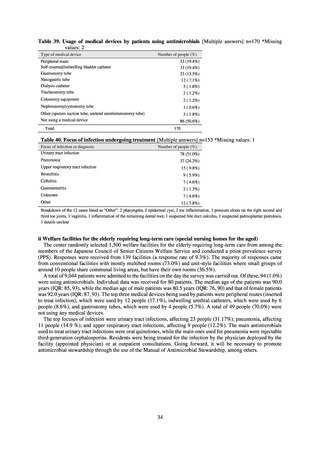
Data on antimicrobial resistance which are considered pertinent from public health perspective or public
awareness toward AMR, but not monitored neither by current suveillance nor monitoring systems were obtained
from findings by Health and Labor Sciences Research Groups.
The results of the survey of attitudes among veterinary science students specializing in the animal field are based
on responses to a questionnaire conducted in conjunction with a lecture on antimicrobial resistance at 12
universities.
Results:
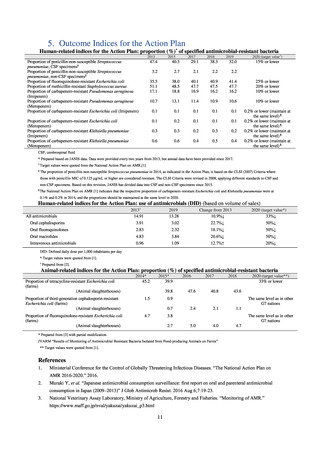
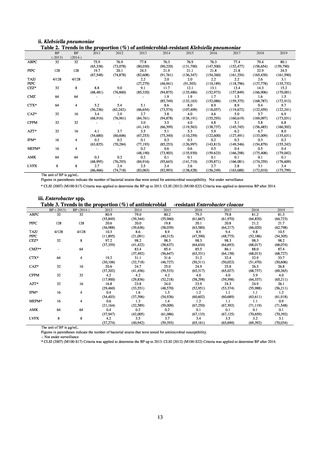
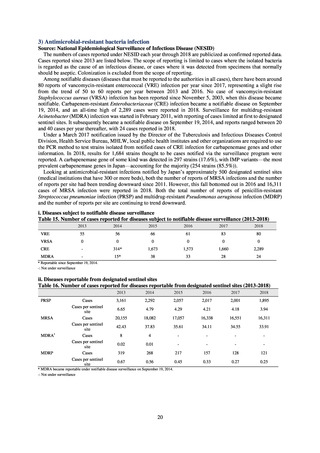
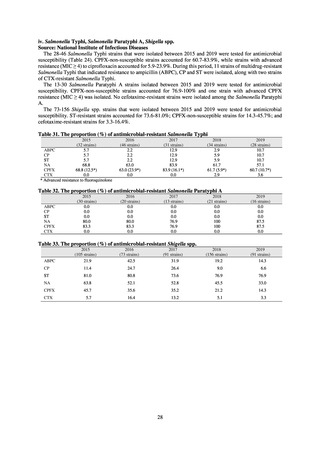
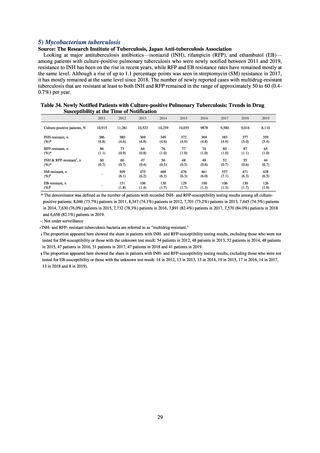
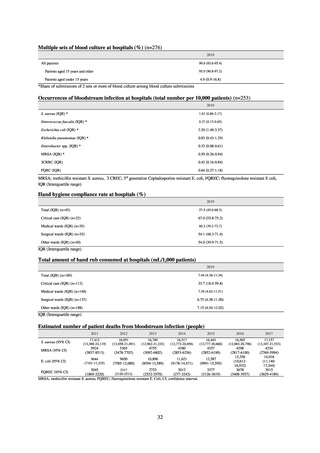
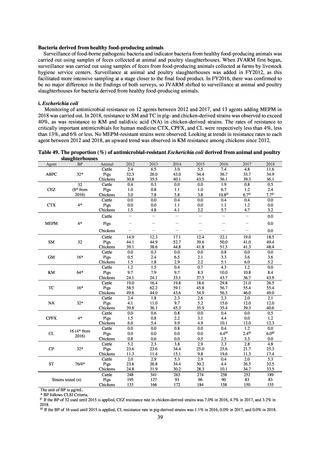
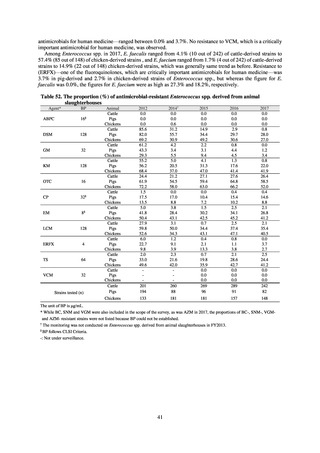
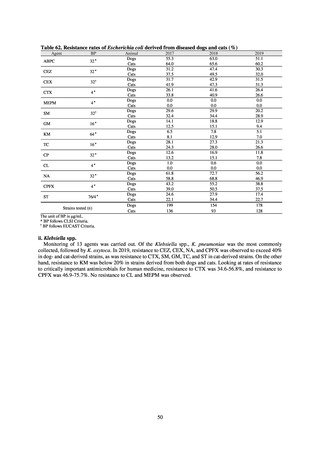
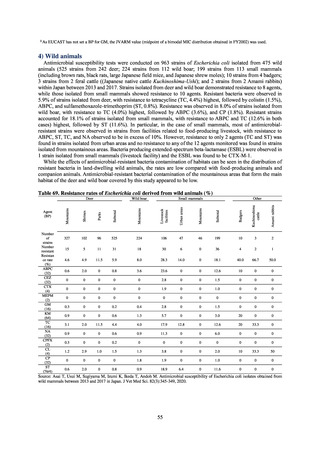
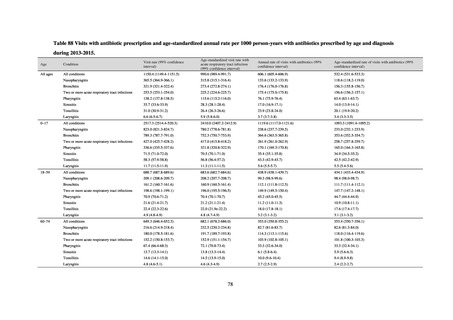
In Japan, the carbapenem resistance rate in Enterobacteriaceae, particularly Escherichia coli and Klebsiella
pneumoniae has remained below 1% during the observed period, despite its global increase in human isolates.
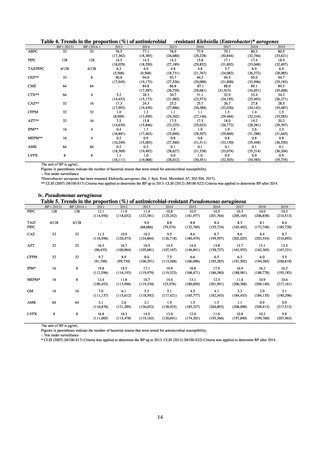
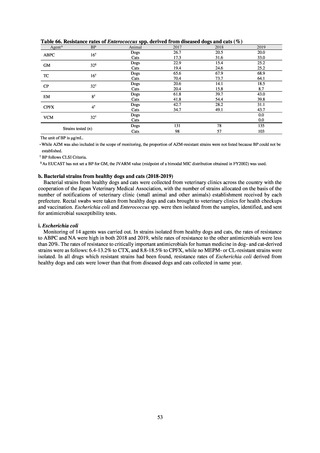
Internationally, the increase in vancomycin resistance among enterococci is a problem. While vancomycin
resistance among Enterococcus faecium in Japan remained less than 1% until 2018, its progress needs to be tracked
carefully, as the figure increased to 1.5% in 2019. While the criteria for assessing carbapenem resistance in
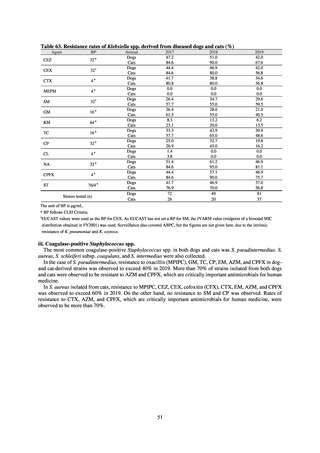
Pseudomonas aeruginosa changed in 2014, the resistance rate appears to be trending downward. The rate of
resistance against the third-generation cephalosporins and fluoroquinolones among Escherichia coli, however, is
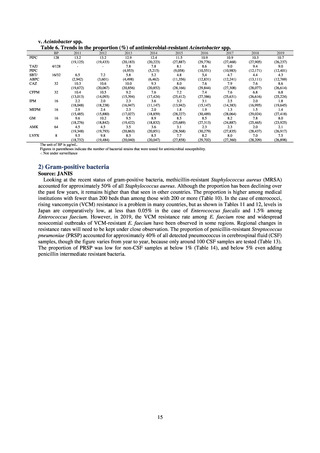
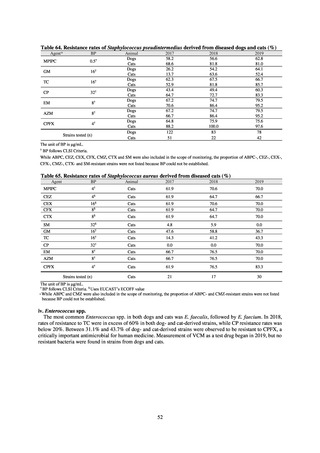
increasing. Although the percentage of methicillin-resistant Staphylococcus aureus (MRSA) has been declining
since 2011, levels remain high. Clear similarities in the pattern of resistance rates to antimicrobials were observed
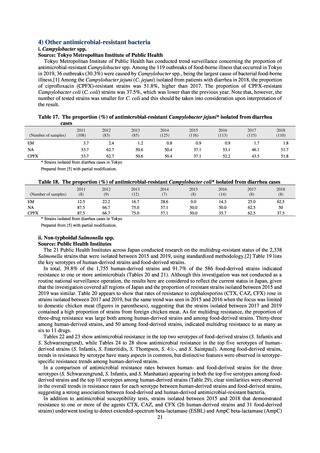
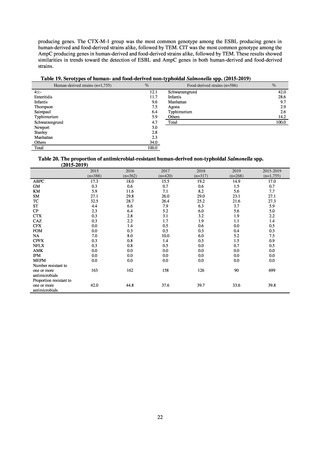
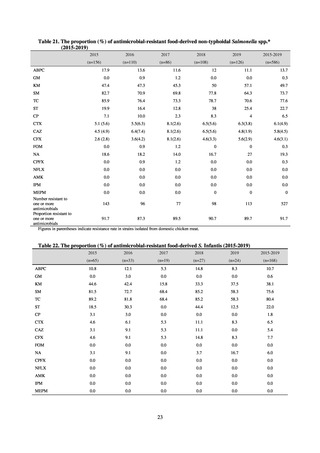
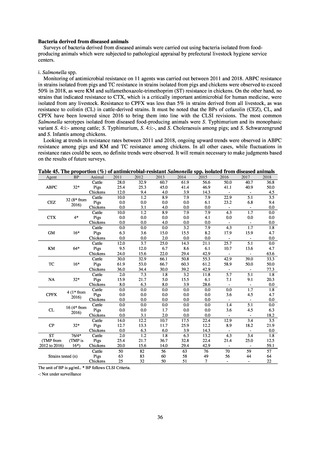
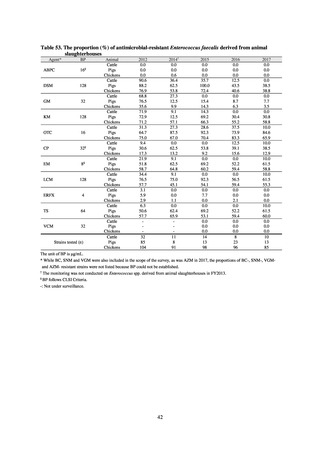
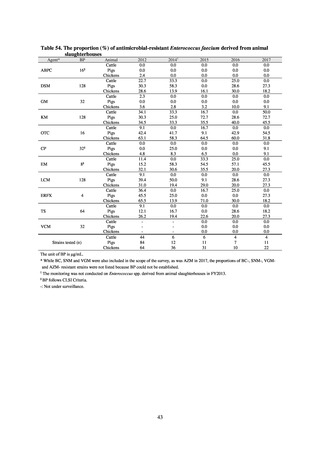
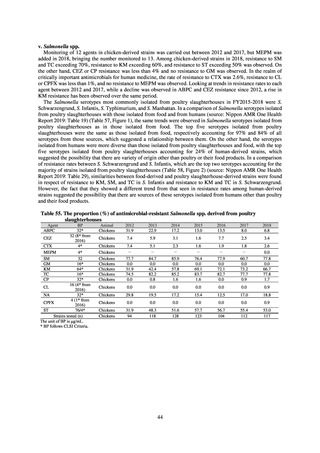
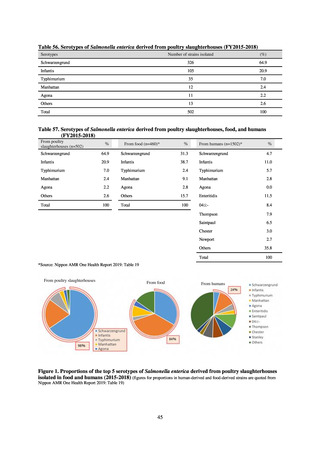
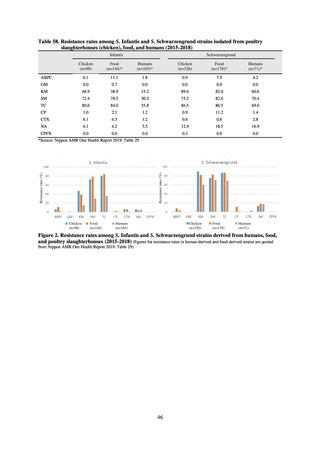
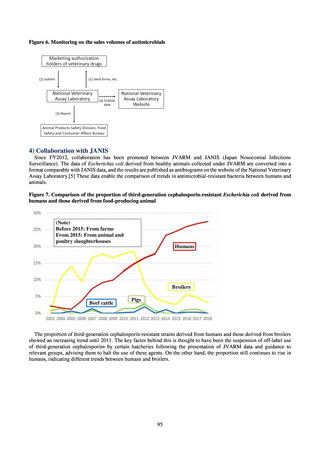
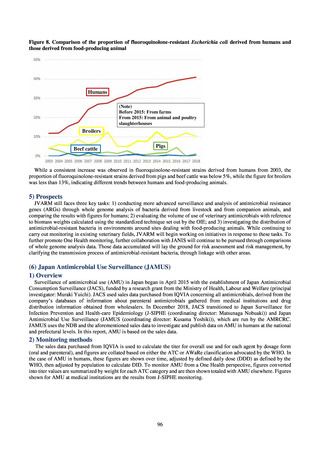
in serotypes of Salmonella spp. isolated from food and from humans, strongly suggesting a link between resistant
strains derived from food and from humans.
8
DDDs per 100 bed-days and DDDs per 1,000 patient-days.
・DID (DDDs/1,000 inhabitants/day): Antimicrobial use in a region or country is expressed as DID, which is
calculated by dividing the total titer by DDD and correcting the denominator with the number of inhabitants of the
region per day.
・DOT (day of therapy): DOT is calculated by correcting the total days of therapy (DOTs) using antimicrobials in a
specified period with the total patient-days. The units used for DOT include DOTs per 100 bed-days and DOTs per
1,000 patient-days.
Executive Summary
Background:
Japan’s “National Action Plan on AMR 2016-2020” positions efforts to ascertain the current status of
antimicrobial-resistant bacteria and national antimicrobial use in the areas of human health, animals, food and the
environment and trends therein as an important strategy for both evaluating current policy and examining future
policy. For global monitoring and reporting, WHO has launched the Global Antimicrobial Resistance Surveillance
System (GLASS) for the gathering and sharing of trends in resistant bacteria worldwide. Japan contributes to
GLASS by providing our national data. In addition, Japan also submits data as part of our assistance with an
initiative by the World Organisation for Animal Health (OIE), which uses standardized methods for monitoring
the volume of antimicrobial use in animals. Accordingly, it is crucial for Japan to update both domestic and
overseas stakeholders about the current status and progress of our AMR policy, in order both to reaffirm Japan’s
position in the global community and to accelerate and advance AMR policy internationally.
Method:
The AMR One Health Surveillance Committee, comprised of experts on AMR in the areas of human health,
animals, food and the environment, discussed current surveillance/monitoring systems and reviewed published
research on AMR and antimicrobial use. Data on the proportion of antimicrobial resistance among major
pathogens in the human medical setting were derived from the Japan Nosocomial Infections Surveillance (JANIS)
program organized by the Ministry of Health, Labour and Welfare of Japan. Data on the proportion of
antimicrobial resistance among animals and related antimicrobial sales were derived from the Japanese Veterinary
Antimicrobial Resistance Monitoring System (JVARM) implemented by the Ministry of Agriculture, Forestry and
Fisheries of Japan (MAFF). We obtained data on sales and consumption of antimicrobials for human use from
IQVIA Solutions Japan K.K., the National Database of Health Insurance Claims and Specific Health Checkups of
Japan (NDB), and Japan Surveillance for Infection Prevention and Health‐care Epidemiology (J-SIPHE). Data on
the distribution of antimicrobial feed additives were provided by the Food and Agricultural Materials Inspection
Center (FAMIC) and the Japan Scientific Feeds Associations (JSFA). Data on the volume of domestic shipments
of antimicrobials used as agricultural chemicals was from MAFF.
Data on antimicrobial resistance which are considered pertinent from public health perspective or public
awareness toward AMR, but not monitored neither by current suveillance nor monitoring systems were obtained
from findings by Health and Labor Sciences Research Groups.
The results of the survey of attitudes among veterinary science students specializing in the animal field are based
on responses to a questionnaire conducted in conjunction with a lecture on antimicrobial resistance at 12
universities.
Results:
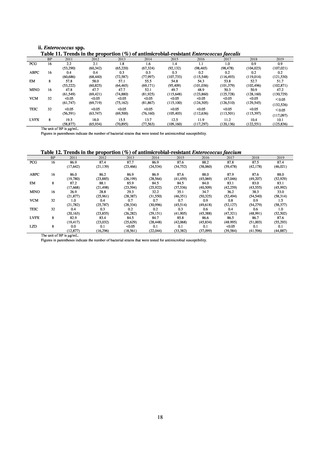
In Japan, the carbapenem resistance rate in Enterobacteriaceae, particularly Escherichia coli and Klebsiella
pneumoniae has remained below 1% during the observed period, despite its global increase in human isolates.
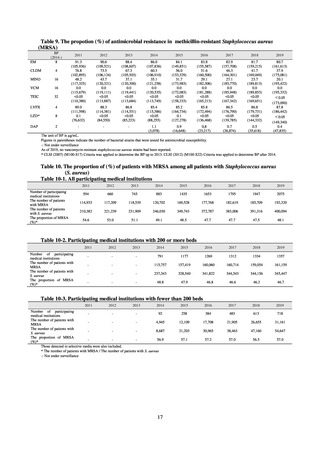
Internationally, the increase in vancomycin resistance among enterococci is a problem. While vancomycin
resistance among Enterococcus faecium in Japan remained less than 1% until 2018, its progress needs to be tracked
carefully, as the figure increased to 1.5% in 2019. While the criteria for assessing carbapenem resistance in
Pseudomonas aeruginosa changed in 2014, the resistance rate appears to be trending downward. The rate of
resistance against the third-generation cephalosporins and fluoroquinolones among Escherichia coli, however, is
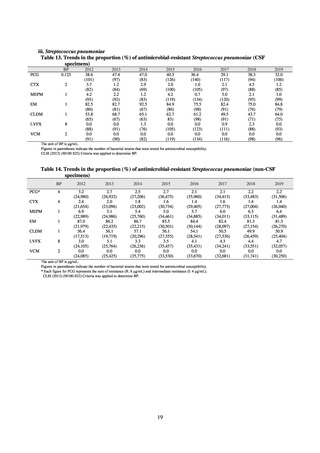
increasing. Although the percentage of methicillin-resistant Staphylococcus aureus (MRSA) has been declining
since 2011, levels remain high. Clear similarities in the pattern of resistance rates to antimicrobials were observed
in serotypes of Salmonella spp. isolated from food and from humans, strongly suggesting a link between resistant
strains derived from food and from humans.
8