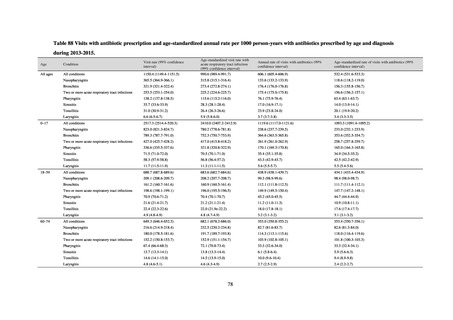
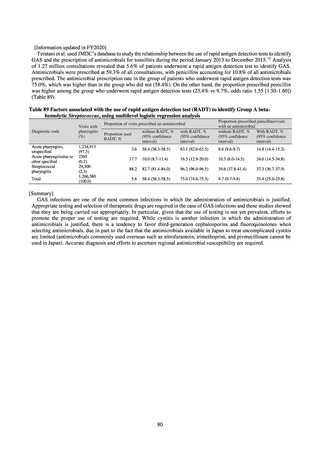
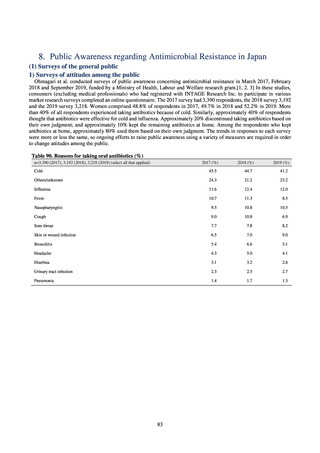
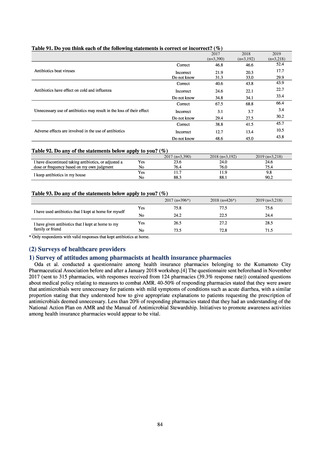
よむ、つかう、まなぶ。
【参考資料3】【英版R4.1.17】Nippon AMR One Health Report (NAOR) 2020 (91 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23261.html |
| 出典情報 | 国際的に脅威となる感染症対策関係閣僚会議 薬剤耐性ワンヘルス動向調査検討会(第9回 1/17)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
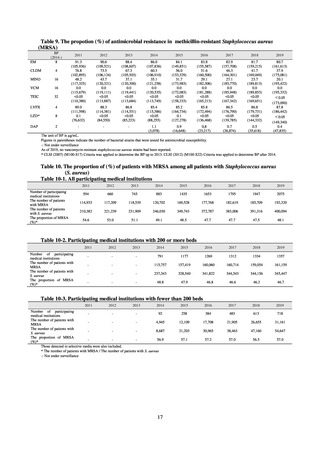
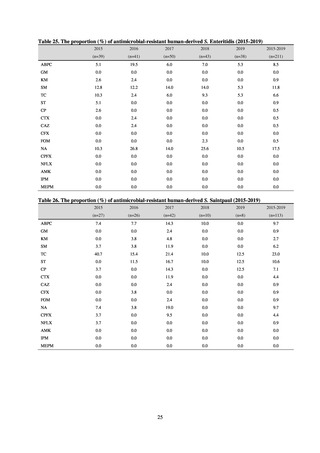
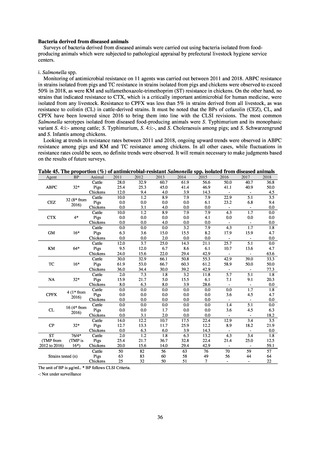
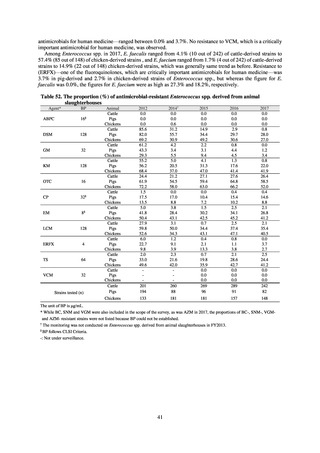
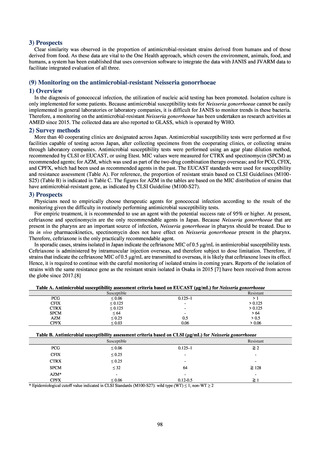
Table A. Reporting criteria
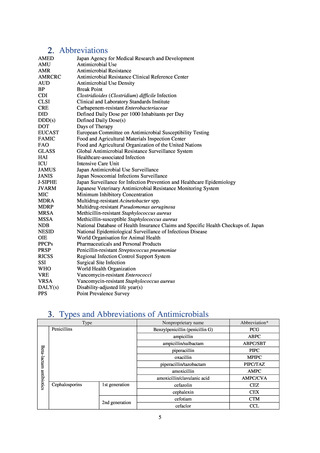
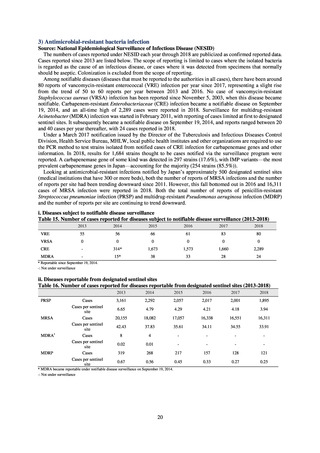
Reportable disease
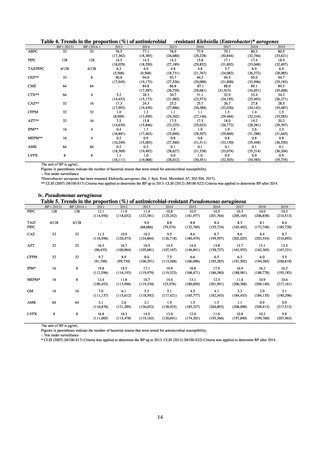
VRE
VRSA
CRE
MDRA
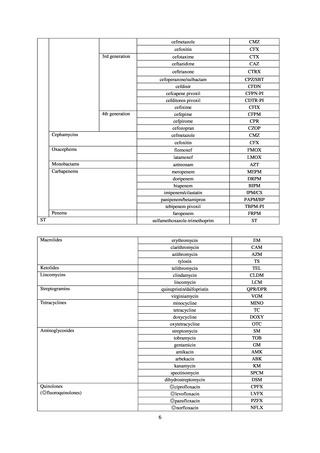
Summary of reporting criteria
Enterococcus is isolated and identified, and the MIC value of vancomycin is ≥ 16 μg/mL.
Staphylococcus aureus is isolated and identified, and the MIC value of vancomycin is ≥ 16 μg/mL.
Enterobacteriaceae is isolated and identified, and either A) or B) below is satisfied:
A) The MIC value of meropenem is ≥ 2 μg/mL,
or the diameter of the inhibition circle of the meropenem susceptibility disk (KB) is ≤ 22 mm.
B) It is confirmed that both the following conditions are satisfied:
a) The MIC value of imipenem is ≥ 2 μg/mL,
or the diameter of the inhibition circle of the imipenem susceptibility disk (KB) is ≤ 22 mm.
b) The MIC value of cefmetazole is ≥ 64 μg/mL,
or the diameter of the inhibition circle of the cefmetazole susceptibility disk (KB) is ≤ 12 mm.
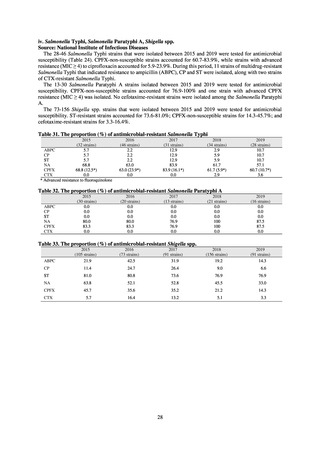
MDRA Acinetobacter spp. is isolated and identified, and all three conditions below are satisfied:
A) The MIC value of imipenem is ≥ 16 μg/mL,
or the diameter of the inhibition circle of the imipenem susceptibility disk (KB) is ≤ 13 mm.
B) The MIC value of amikacin is ≥ 32 μg/mL,
or the diameter of the inhibition circle of the amikacin susceptibility disk (KB) is ≤ 14 mm.
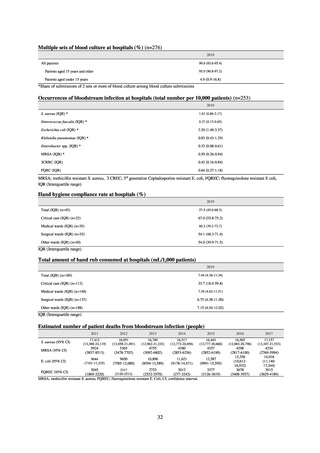
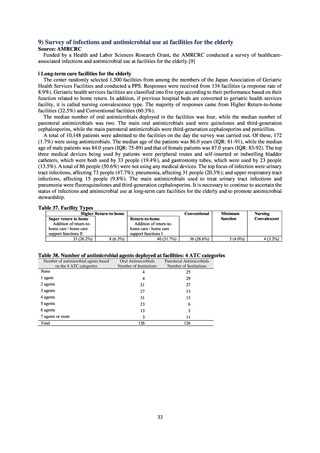
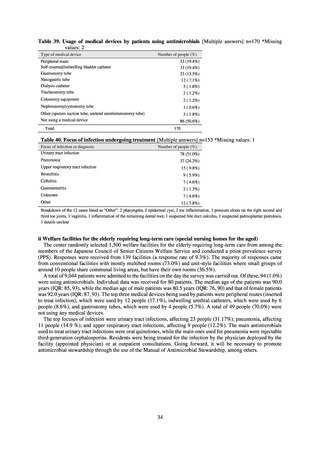
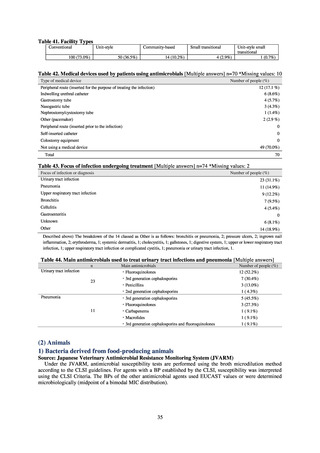
C) The MIC value of ciprofloxacin is ≥ 4 μg/mL,
or the diameter of the inhibition circle of the ciprofloxacin susceptibility disk (KB) is ≤ 15 mm.
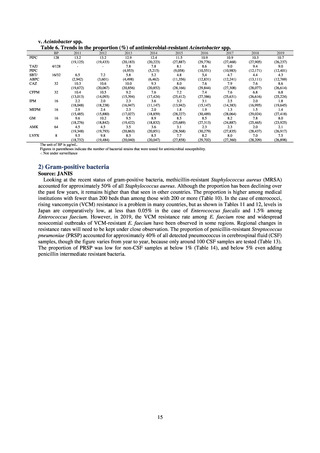
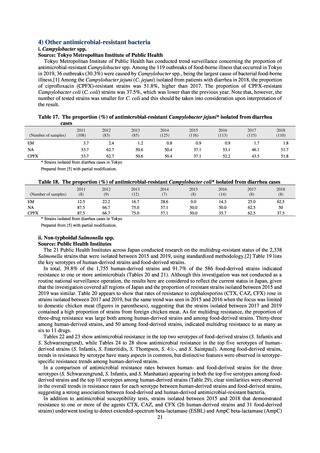
PRSP
Streptococcus pneumoniae is isolated and identified, and the MIC value of penicillin is ≥ 0.125 μg/mL,
or the diameter of the inhibition circle of the oxacillin susceptibility disk (KB) is ≤ 19 mm.
MRSA
Staphylococcus aureus is isolated and identified, and the MIC value of oxacillin is ≥ 4 μg/mL,
or the diameter of the inhibition circle of the oxacillin susceptibility disk (KB) is ≤ 10 mm.
MDRP
Pseudomonas aeruginosa is isolated and identified, and all three conditions below are satisfied:
A) The MIC value of imipenem is ≥ 16 μg/mL,
or the diameter of the inhibition circle of the imipenem susceptibility disk (KB) is ≤ 13 mm.
B) The MIC value of amikacin is ≥ 32 μg/mL,
or the diameter of the inhibition circle of the amikacin susceptibility disk (KB) is ≤ 14 mm.
C) The MIC value of ciprofloxacin is ≥ 4 μg/mL,
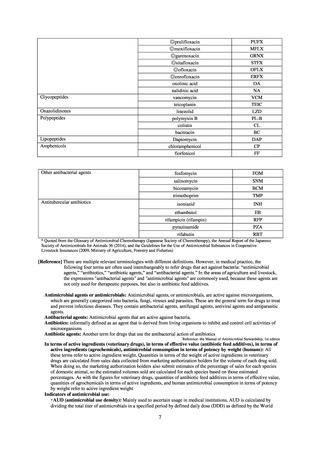
or the diameter of the inhibition circle of the ciprofloxacin susceptibility disk (KB) is ≤ 15 mm.
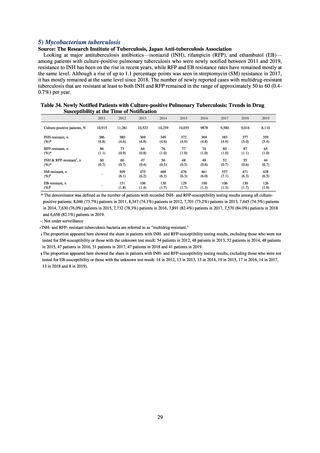
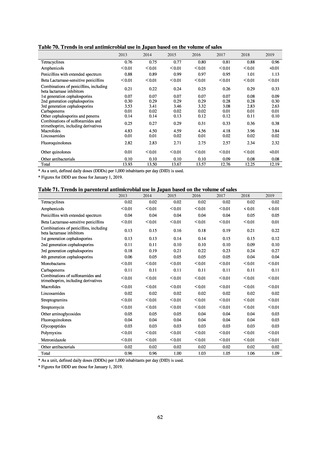
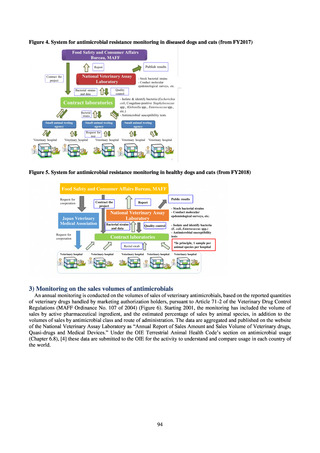
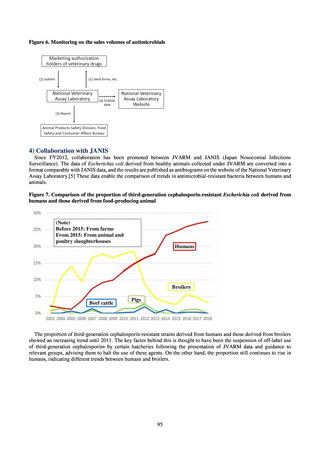
3) System
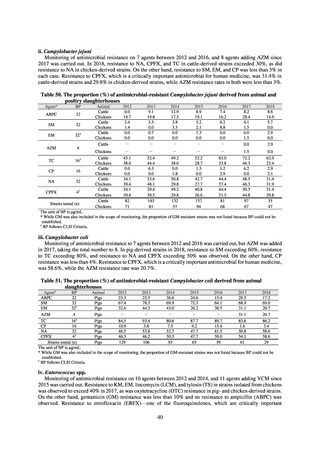
Public Health Centers confirm reported information, and enter the data into NESID. The registered information is further
confirmed and analyzed, and additional information is collected, by local infectious disease surveillance centers, the
Infectious Diseases Surveillance Center of NIID as the central infectious disease surveillance center, and other relevant
bodies. Patient information (e.g. the reported numbers of patients, and trends) that is collected under the Infectious Diseases
Control Law, and other related information, are provided to the general public through the Infectious Diseases Weekly
Reports (IDWRs) and other media. A March 2017 notification issued by the Director of the Tuberculosis and Infectious
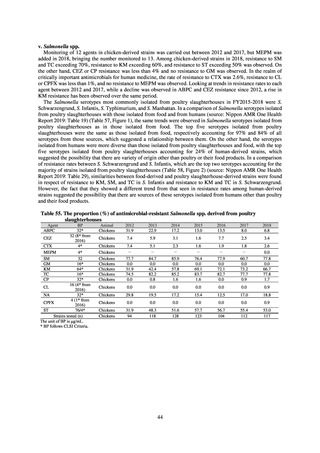
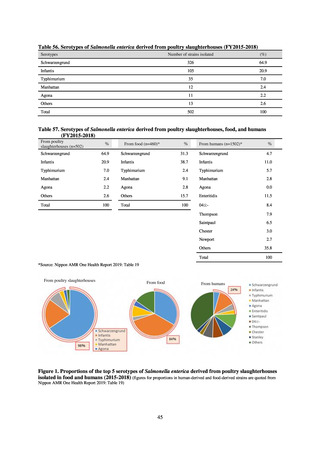
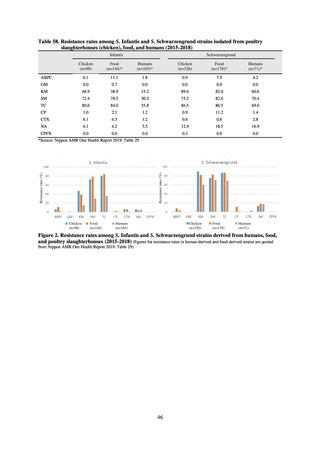
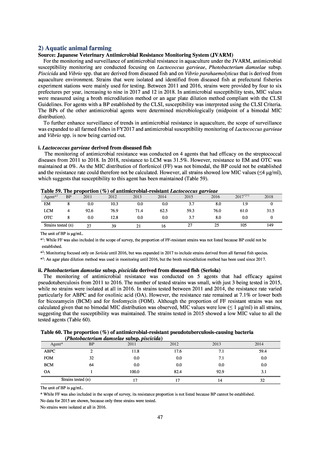
Diseases Control Division, Health Service Bureau, MHLW imposed on local public health institutes and other
organizations a requirement to test strains isolated from notified cases of CRE infection. Since then, data concerning the
detection of major carbapenemase genes in strains isolated from notified cases of CRE infection have been collected and
analyzed within the framework of the monitoring of trends in outbreaks of infection and have been published in the
Infectious Agents Surveillance Report (IASR), among others.
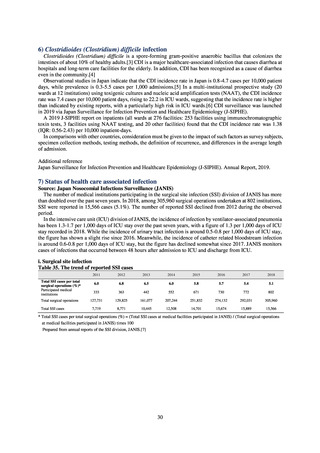
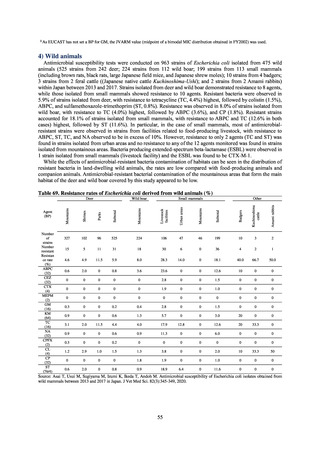
4) Prospects
A certain level of quality is considered to be guaranteed in the reporting of antimicrobial-resistant bacteria infections
under NESID, since reporting is based on case definitions specified by the Infectious Diseases Control Law. Although
cases may be underestimated in notifiable disease surveillance, an overall picture of trends in occurrence can be monitored.
This surveillance system is also considered useful because, when an unusual trend is observed, it may trigger an intervention
(e.g. investigation, guidance) at the relevant medical institution by the Public Health Center. Trends in diseases reportable
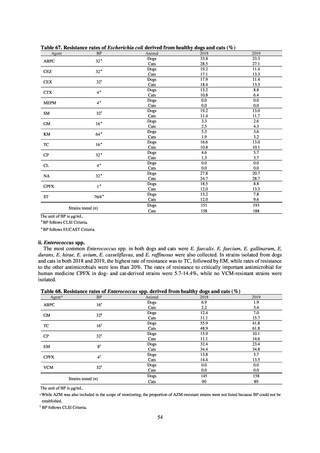
from designated sentinel sites have been recorded since the launch of the NESID program in 1999, and considered useful
for monitoring medium- to long-term trends in the occurrence of the target diseases. In addition, pathogen surveillance
focused primarily on CRE was launched in 2017 and, with data on resistance genes set to be gathered and analyzed for
VRE and MDRA in due course, it is anticipated that information that will be valuable in devising measures to combat
antimicrobial-resistant bacteria will be collected and utilized.
90
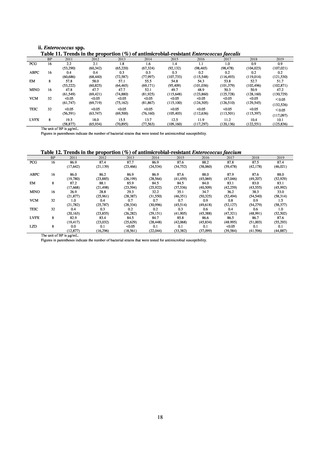
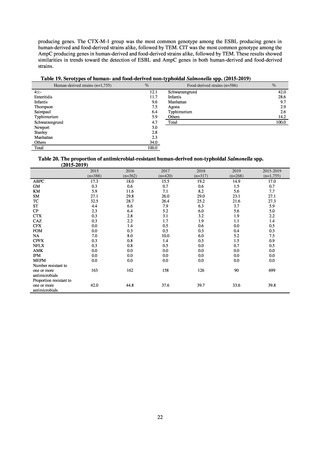
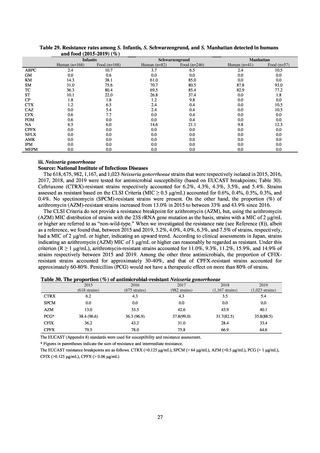
Reportable disease
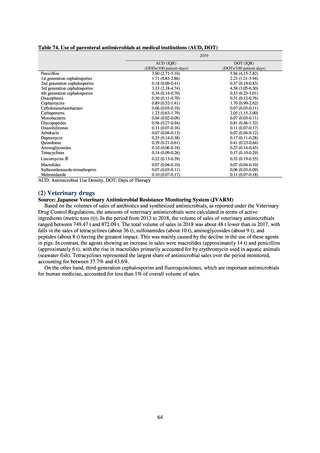
VRE
VRSA
CRE
MDRA
Summary of reporting criteria
Enterococcus is isolated and identified, and the MIC value of vancomycin is ≥ 16 μg/mL.
Staphylococcus aureus is isolated and identified, and the MIC value of vancomycin is ≥ 16 μg/mL.
Enterobacteriaceae is isolated and identified, and either A) or B) below is satisfied:
A) The MIC value of meropenem is ≥ 2 μg/mL,
or the diameter of the inhibition circle of the meropenem susceptibility disk (KB) is ≤ 22 mm.
B) It is confirmed that both the following conditions are satisfied:
a) The MIC value of imipenem is ≥ 2 μg/mL,
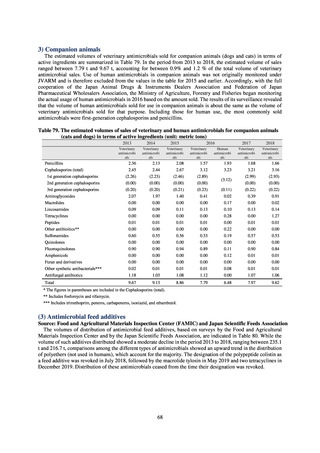
or the diameter of the inhibition circle of the imipenem susceptibility disk (KB) is ≤ 22 mm.
b) The MIC value of cefmetazole is ≥ 64 μg/mL,
or the diameter of the inhibition circle of the cefmetazole susceptibility disk (KB) is ≤ 12 mm.
MDRA Acinetobacter spp. is isolated and identified, and all three conditions below are satisfied:
A) The MIC value of imipenem is ≥ 16 μg/mL,
or the diameter of the inhibition circle of the imipenem susceptibility disk (KB) is ≤ 13 mm.
B) The MIC value of amikacin is ≥ 32 μg/mL,
or the diameter of the inhibition circle of the amikacin susceptibility disk (KB) is ≤ 14 mm.
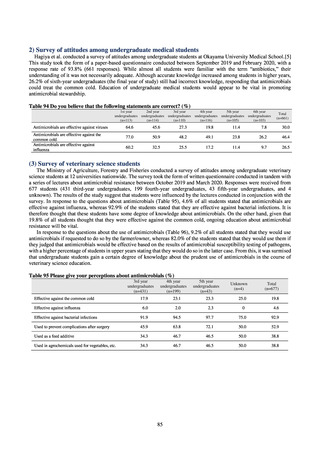
C) The MIC value of ciprofloxacin is ≥ 4 μg/mL,
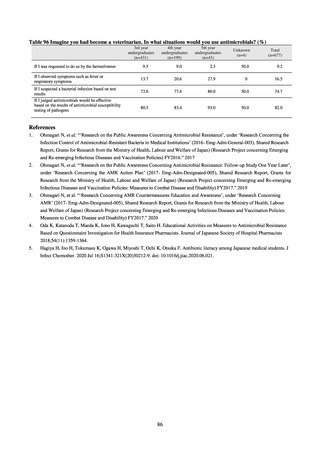
or the diameter of the inhibition circle of the ciprofloxacin susceptibility disk (KB) is ≤ 15 mm.
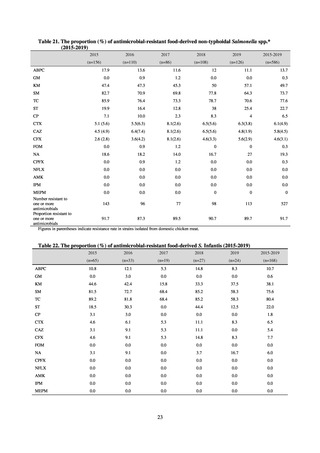
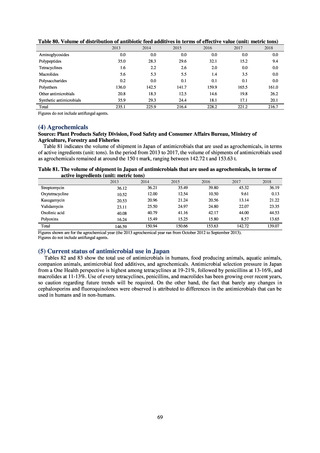
PRSP
Streptococcus pneumoniae is isolated and identified, and the MIC value of penicillin is ≥ 0.125 μg/mL,
or the diameter of the inhibition circle of the oxacillin susceptibility disk (KB) is ≤ 19 mm.
MRSA
Staphylococcus aureus is isolated and identified, and the MIC value of oxacillin is ≥ 4 μg/mL,
or the diameter of the inhibition circle of the oxacillin susceptibility disk (KB) is ≤ 10 mm.
MDRP
Pseudomonas aeruginosa is isolated and identified, and all three conditions below are satisfied:
A) The MIC value of imipenem is ≥ 16 μg/mL,
or the diameter of the inhibition circle of the imipenem susceptibility disk (KB) is ≤ 13 mm.
B) The MIC value of amikacin is ≥ 32 μg/mL,
or the diameter of the inhibition circle of the amikacin susceptibility disk (KB) is ≤ 14 mm.
C) The MIC value of ciprofloxacin is ≥ 4 μg/mL,
or the diameter of the inhibition circle of the ciprofloxacin susceptibility disk (KB) is ≤ 15 mm.
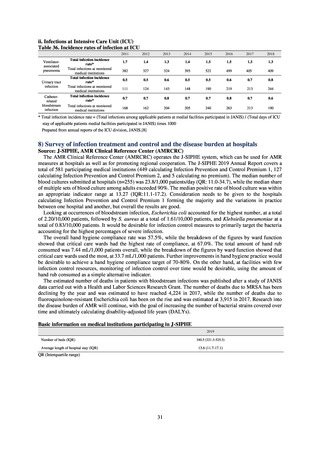
3) System
Public Health Centers confirm reported information, and enter the data into NESID. The registered information is further
confirmed and analyzed, and additional information is collected, by local infectious disease surveillance centers, the
Infectious Diseases Surveillance Center of NIID as the central infectious disease surveillance center, and other relevant
bodies. Patient information (e.g. the reported numbers of patients, and trends) that is collected under the Infectious Diseases
Control Law, and other related information, are provided to the general public through the Infectious Diseases Weekly
Reports (IDWRs) and other media. A March 2017 notification issued by the Director of the Tuberculosis and Infectious
Diseases Control Division, Health Service Bureau, MHLW imposed on local public health institutes and other
organizations a requirement to test strains isolated from notified cases of CRE infection. Since then, data concerning the
detection of major carbapenemase genes in strains isolated from notified cases of CRE infection have been collected and
analyzed within the framework of the monitoring of trends in outbreaks of infection and have been published in the
Infectious Agents Surveillance Report (IASR), among others.
4) Prospects
A certain level of quality is considered to be guaranteed in the reporting of antimicrobial-resistant bacteria infections
under NESID, since reporting is based on case definitions specified by the Infectious Diseases Control Law. Although
cases may be underestimated in notifiable disease surveillance, an overall picture of trends in occurrence can be monitored.
This surveillance system is also considered useful because, when an unusual trend is observed, it may trigger an intervention
(e.g. investigation, guidance) at the relevant medical institution by the Public Health Center. Trends in diseases reportable
from designated sentinel sites have been recorded since the launch of the NESID program in 1999, and considered useful
for monitoring medium- to long-term trends in the occurrence of the target diseases. In addition, pathogen surveillance
focused primarily on CRE was launched in 2017 and, with data on resistance genes set to be gathered and analyzed for
VRE and MDRA in due course, it is anticipated that information that will be valuable in devising measures to combat
antimicrobial-resistant bacteria will be collected and utilized.
90