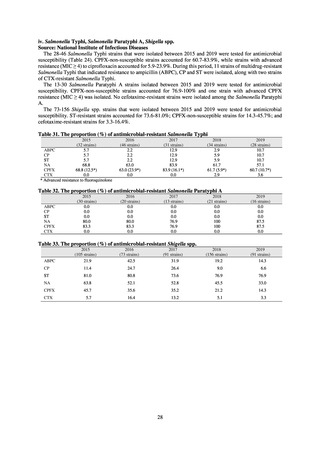
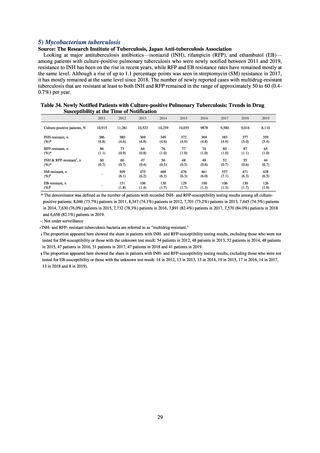
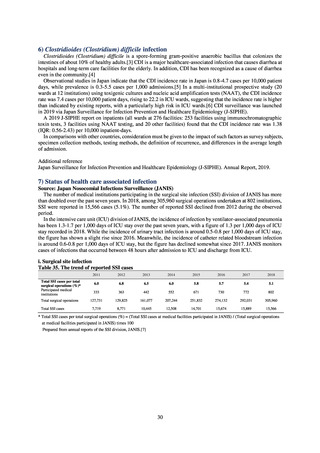
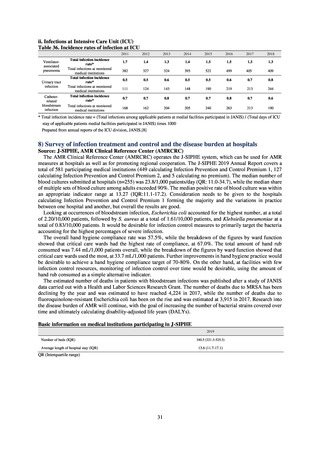
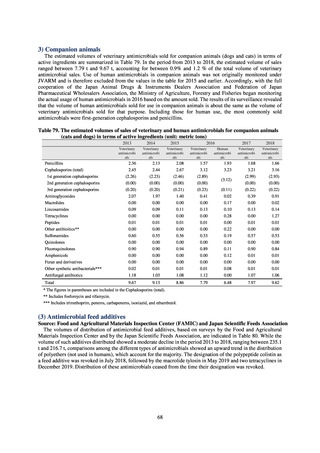
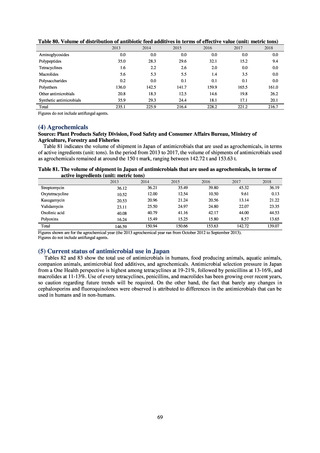
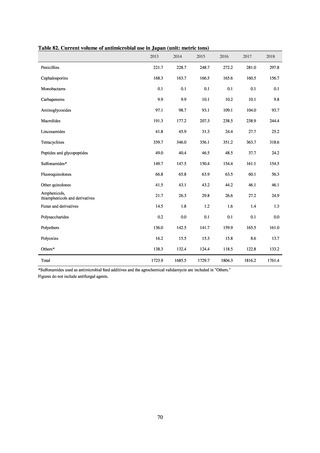
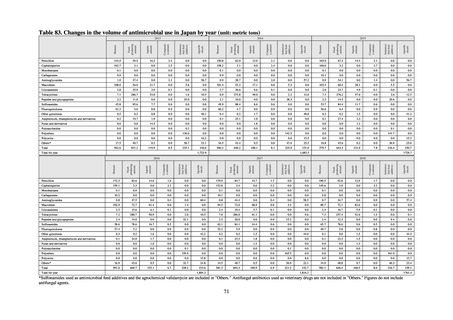
よむ、つかう、まなぶ。
【参考資料3】【英版R4.1.17】Nippon AMR One Health Report (NAOR) 2020 (89 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23261.html |
| 出典情報 | 国際的に脅威となる感染症対策関係閣僚会議 薬剤耐性ワンヘルス動向調査検討会(第9回 1/17)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
Appendix
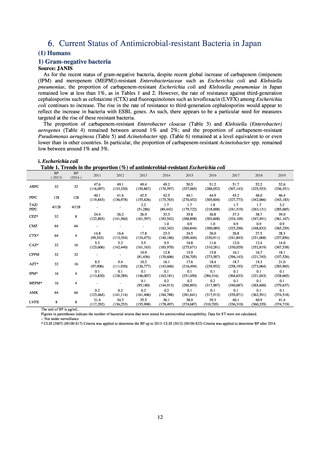
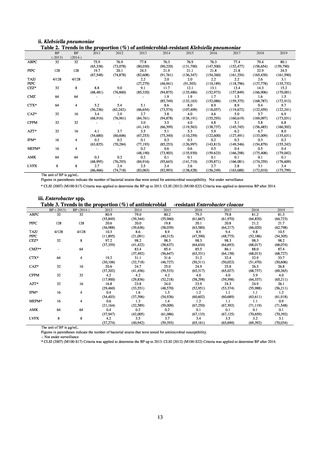
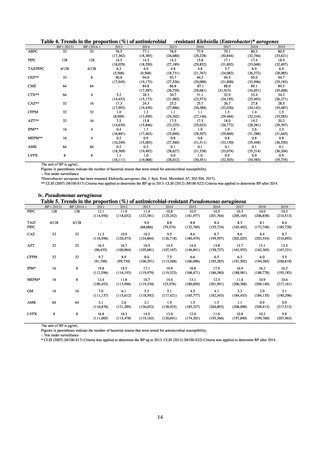
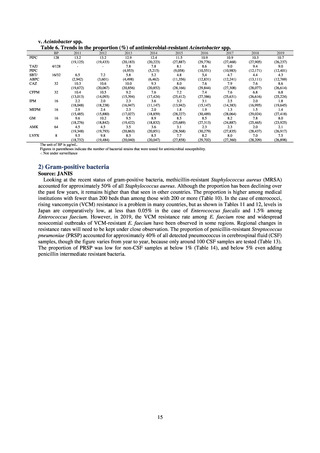
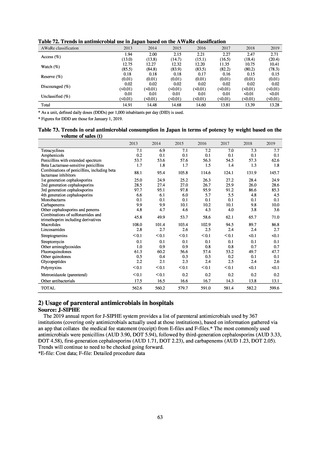
(1) Japan Nosocomial Infections Surveillance (JANIS)
1) Overview
JANIS is conducted for the purpose of having an overview of nosocomial infections in Japan, by surveying the status of
health care associated infections at medical institutions in Japan, the isolation of antimicrobial-resistant bacteria, and the
status of infections caused by antimicrobial-resistant bacteria, while providing useful information for the control of health
care associated infections in medical settings. The aggregated data of information from all medical institutions patriated
are published on the website of the National Institute of Infectious Diseases (https://janis.mhlw.go.jp/english/index.asp). A
result of the analysis is reported back to each institution so that such a feedback can be utilized for the formulation and
evaluation of infection control measures at each institution. JANIS participation is voluntary with approximately 2,000
participating medical institutions at present.
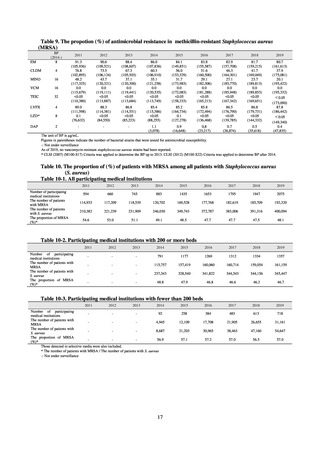
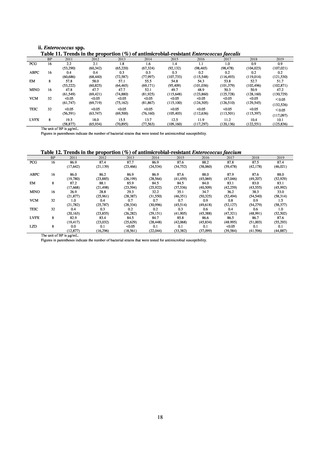
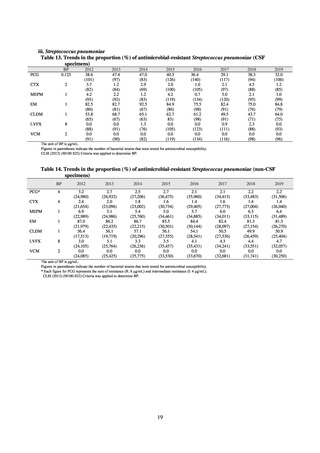
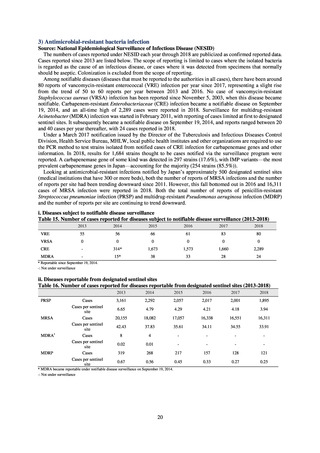
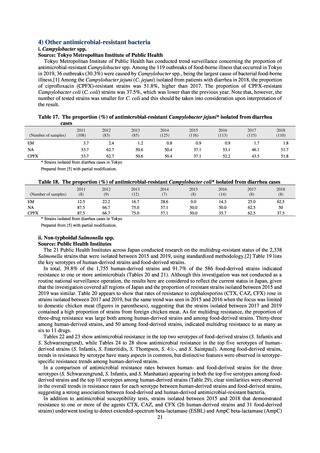
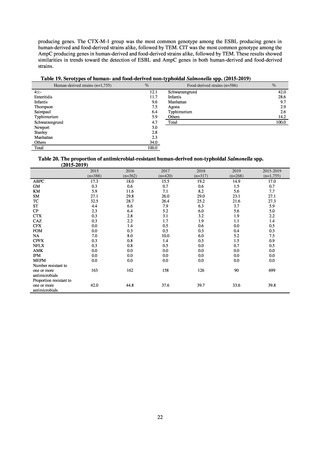
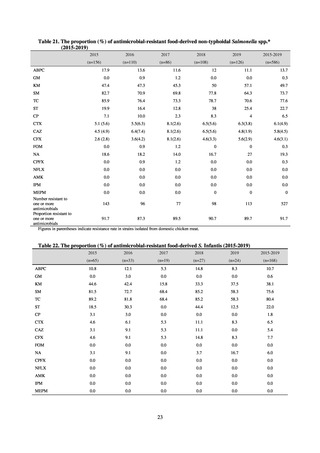
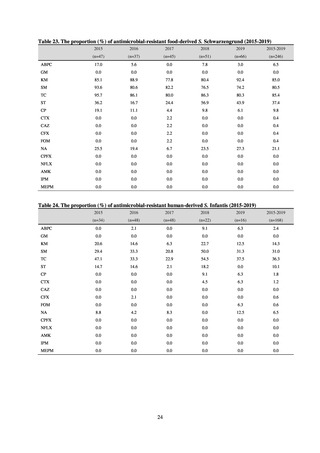
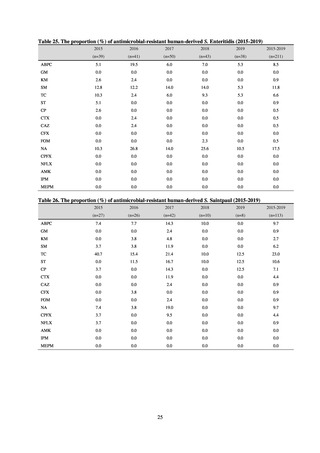
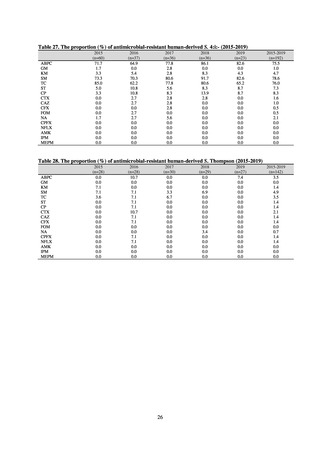
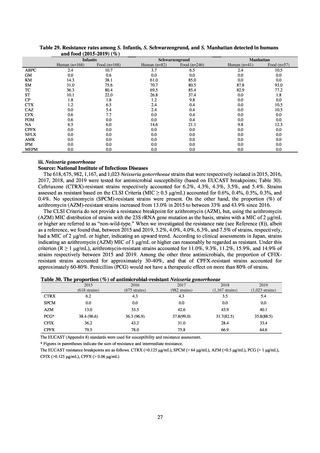
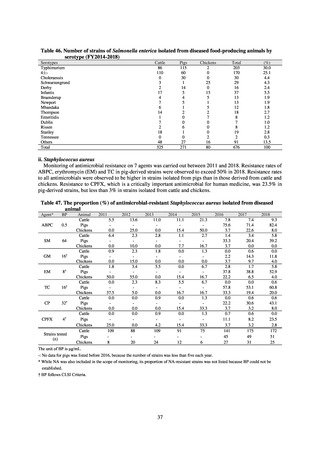
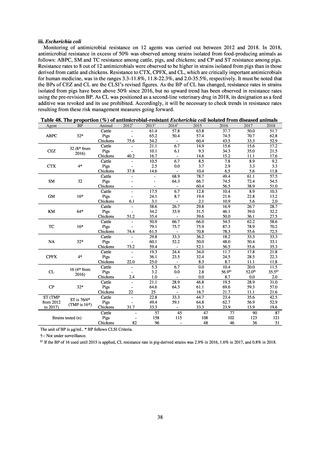
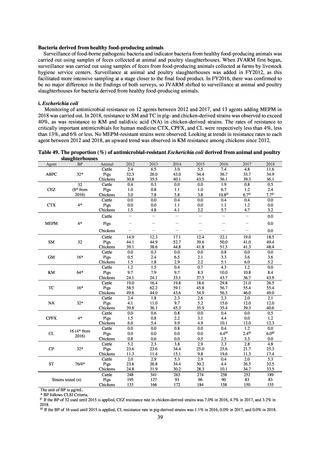
Clinical Laboratory Division of JANIS collects the laboratory data of bacteria that are isolated at hospitals across Japan,
and publish aggregated data regarding the proportion of clinically important bacterial species that are resistant to major
antimicrobials. In 2018, 1,988 hospitals participated in the laboratory section. The aggregated data include data from
hospitals with at least 20 beds, and exclude clinics and facilities for the elderly. Since 2014, figures have also been compiled
on the basis of hospital scale, divided into hospitals with 200 or more beds and those with fewer than 200 beds. Only
bacteria that are isolated from specimens from hospitalized patients at participating hospitals are included into aggregated
data, and specimens from ambulatory sections are excluded. To provide more representative information as a national
surveillance system, protocols of sampling including selection of sentinel sites and their stratification need to be improved
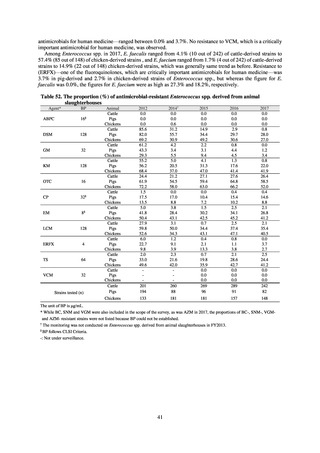
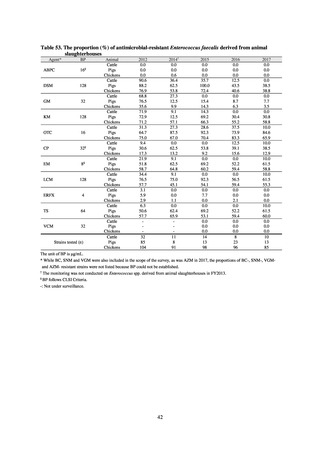
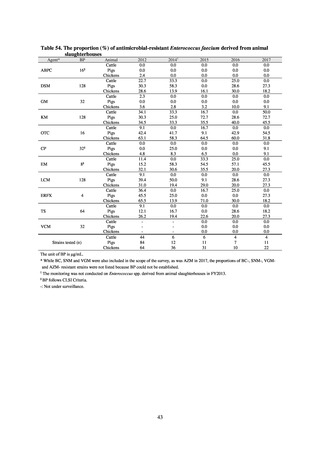
further. The assessment of antimicrobial susceptibility tests is interpreted based on CLSI Criteria.
Quality control for antimicrobial susceptibility tests depends on medical institutions. To improve the quality of
antimicrobial susceptibility tests at hospital laboratories, a quality control program was developed under the leadership of
the Japanese Society for Clinical Microbiology and it has been piloted since 2016.
JANIS is a surveillance program regulated by the Statistics Act and it differs from the National Epidemiological
Surveillance of Infectious Diseases based on the Infectious Diseases Control Act. While participation is voluntary, from
2014, Premiums for infection control 1 in medical reimbursement requires participation in JANIS or equivalent surveillance
programs. JANIS is organized and operated by the Ministry of Health, Labour and Welfare, and its operating policy is
determined at the operation council that comprises of experts in infectious diseases, antimicrobial resistance and other
relevant professional fields. Section II, Laboratory of Antimicrobial Resistance Surveillance, National Institute of
Infectious Diseases functions as a secretariat office for JANIS.
Under the Global Antimicrobial Resistance Surveillance System (GLASS), launched by WHO in 2015, individual
countries are encouraged to submit data regarding resistant bacterias in the human health area.[1] Japan has provided
necessary data from JANIS and other pertinent monitoring systems to GLASS. Of note, data for 2014 to 2017 have already
been submitted. GLASS is calling for the same set of antimicrobials to be used in antimicrobial susceptibility tests at
medical institutions subject to monitoring in each country. As JANIS is a voluntary surveillance program, it collects
whatever data can be supplied by the participating medical institutions, in whatever form that data emerges from the
institutions’ routine testing operations. Standardizing the types of antimicrobials tested is therefore difficult. Techniques
for compiling data are being considered as part of the JANIS program, to facilitate international cooperation in surveillance.
Under GLASS, the expansion of the scope of surveillance to food-producing animal and other areas are discussed.[1] It is
expected that the data from this national one health report can be contributed to GLASS.
2) Methods for submission
JANIS consists of five divisions: (1) Clinical Laboratory, (2) Antimicrobial-Resistant Bacterial Infection, (3) SSI, (4)
ICU and (5) NICU. Medical institutions select divisions to participate in, in accordance with their purposes and conditions.
Among the five divisions, Clinical Laboratory division handles surveillance regarding antimicrobial resistance. In Clinical
Laboratory division, all data concerning isolated bacteria are collected from bacteriological examination units installed in
the laboratories of medical institutions, computerized systems, and other sources, and converted into the JANIS format
before submitted online. The submitted data are aggregated, and the shares of clinically important bacterial species that are
resistant to key antimicrobials are calculated, and published as the national data of Japan.
3) Prospects
Most medical institutions participating in JANIS are of a relatively large scale with 200 or more beds. The data in the
laboratory division only include specimens from hospitalized patients, and exclude specimens from ambulatory sections.
Data are not collected from clinics. The bias based on this sampling policy in JANIS should be addressed.
88
(1) Japan Nosocomial Infections Surveillance (JANIS)
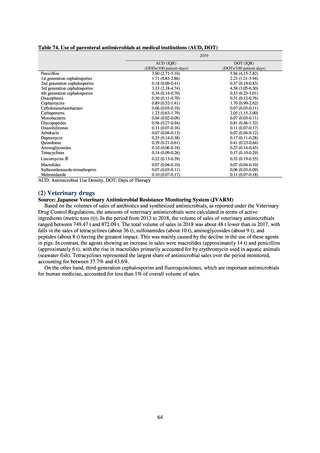
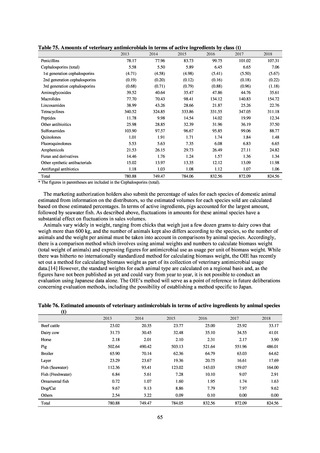
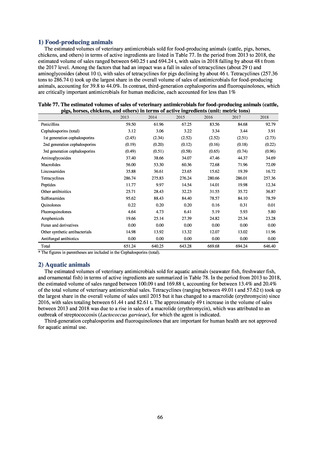
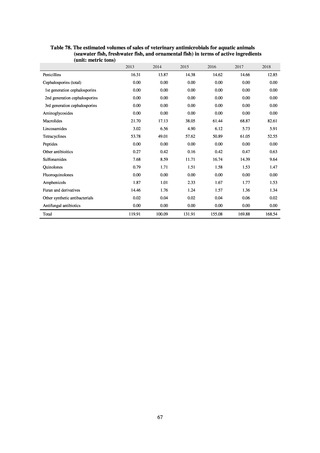
1) Overview
JANIS is conducted for the purpose of having an overview of nosocomial infections in Japan, by surveying the status of
health care associated infections at medical institutions in Japan, the isolation of antimicrobial-resistant bacteria, and the
status of infections caused by antimicrobial-resistant bacteria, while providing useful information for the control of health
care associated infections in medical settings. The aggregated data of information from all medical institutions patriated
are published on the website of the National Institute of Infectious Diseases (https://janis.mhlw.go.jp/english/index.asp). A
result of the analysis is reported back to each institution so that such a feedback can be utilized for the formulation and
evaluation of infection control measures at each institution. JANIS participation is voluntary with approximately 2,000
participating medical institutions at present.
Clinical Laboratory Division of JANIS collects the laboratory data of bacteria that are isolated at hospitals across Japan,
and publish aggregated data regarding the proportion of clinically important bacterial species that are resistant to major
antimicrobials. In 2018, 1,988 hospitals participated in the laboratory section. The aggregated data include data from
hospitals with at least 20 beds, and exclude clinics and facilities for the elderly. Since 2014, figures have also been compiled
on the basis of hospital scale, divided into hospitals with 200 or more beds and those with fewer than 200 beds. Only
bacteria that are isolated from specimens from hospitalized patients at participating hospitals are included into aggregated
data, and specimens from ambulatory sections are excluded. To provide more representative information as a national
surveillance system, protocols of sampling including selection of sentinel sites and their stratification need to be improved
further. The assessment of antimicrobial susceptibility tests is interpreted based on CLSI Criteria.
Quality control for antimicrobial susceptibility tests depends on medical institutions. To improve the quality of
antimicrobial susceptibility tests at hospital laboratories, a quality control program was developed under the leadership of
the Japanese Society for Clinical Microbiology and it has been piloted since 2016.
JANIS is a surveillance program regulated by the Statistics Act and it differs from the National Epidemiological
Surveillance of Infectious Diseases based on the Infectious Diseases Control Act. While participation is voluntary, from
2014, Premiums for infection control 1 in medical reimbursement requires participation in JANIS or equivalent surveillance
programs. JANIS is organized and operated by the Ministry of Health, Labour and Welfare, and its operating policy is
determined at the operation council that comprises of experts in infectious diseases, antimicrobial resistance and other
relevant professional fields. Section II, Laboratory of Antimicrobial Resistance Surveillance, National Institute of
Infectious Diseases functions as a secretariat office for JANIS.
Under the Global Antimicrobial Resistance Surveillance System (GLASS), launched by WHO in 2015, individual
countries are encouraged to submit data regarding resistant bacterias in the human health area.[1] Japan has provided
necessary data from JANIS and other pertinent monitoring systems to GLASS. Of note, data for 2014 to 2017 have already
been submitted. GLASS is calling for the same set of antimicrobials to be used in antimicrobial susceptibility tests at
medical institutions subject to monitoring in each country. As JANIS is a voluntary surveillance program, it collects
whatever data can be supplied by the participating medical institutions, in whatever form that data emerges from the
institutions’ routine testing operations. Standardizing the types of antimicrobials tested is therefore difficult. Techniques
for compiling data are being considered as part of the JANIS program, to facilitate international cooperation in surveillance.
Under GLASS, the expansion of the scope of surveillance to food-producing animal and other areas are discussed.[1] It is
expected that the data from this national one health report can be contributed to GLASS.
2) Methods for submission
JANIS consists of five divisions: (1) Clinical Laboratory, (2) Antimicrobial-Resistant Bacterial Infection, (3) SSI, (4)
ICU and (5) NICU. Medical institutions select divisions to participate in, in accordance with their purposes and conditions.
Among the five divisions, Clinical Laboratory division handles surveillance regarding antimicrobial resistance. In Clinical
Laboratory division, all data concerning isolated bacteria are collected from bacteriological examination units installed in
the laboratories of medical institutions, computerized systems, and other sources, and converted into the JANIS format
before submitted online. The submitted data are aggregated, and the shares of clinically important bacterial species that are
resistant to key antimicrobials are calculated, and published as the national data of Japan.
3) Prospects
Most medical institutions participating in JANIS are of a relatively large scale with 200 or more beds. The data in the
laboratory division only include specimens from hospitalized patients, and exclude specimens from ambulatory sections.
Data are not collected from clinics. The bias based on this sampling policy in JANIS should be addressed.
88