よむ、つかう、まなぶ。
【参考資料3】【英版R4.1.17】Nippon AMR One Health Report (NAOR) 2020 (62 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23261.html |
| 出典情報 | 国際的に脅威となる感染症対策関係閣僚会議 薬剤耐性ワンヘルス動向調査検討会(第9回 1/17)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
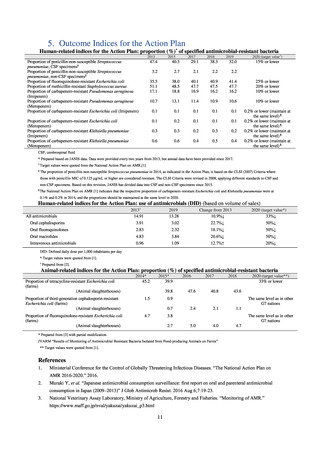
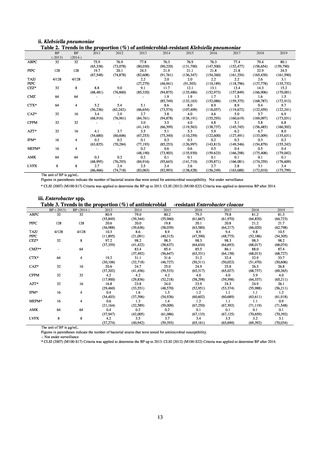
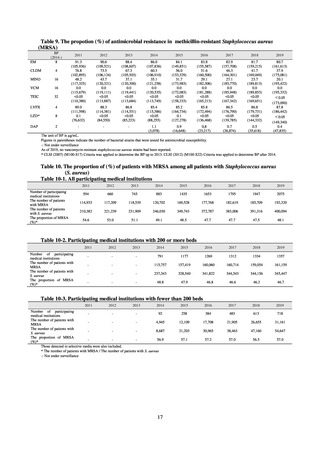
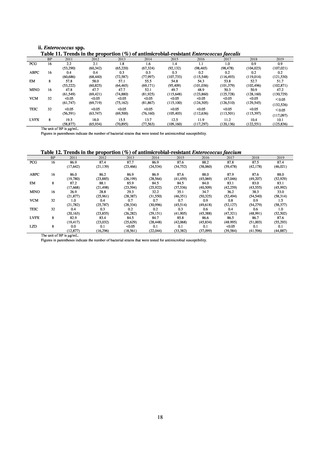
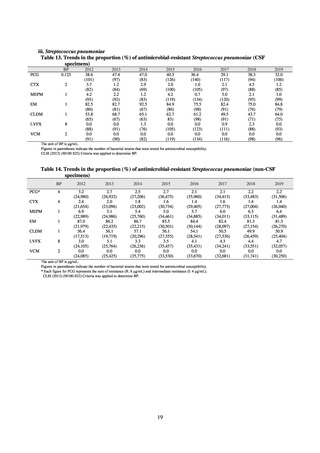
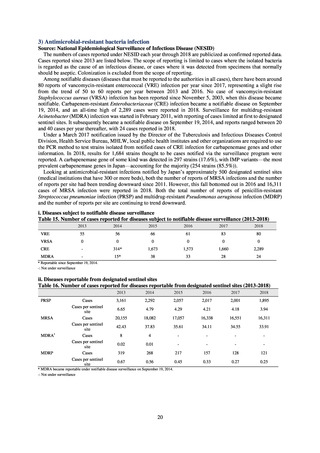
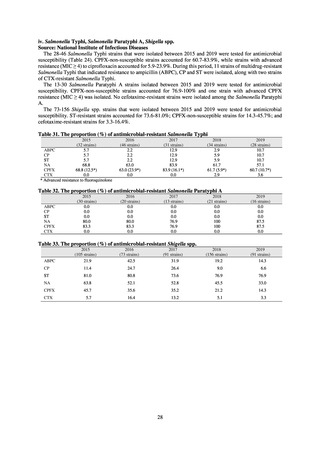
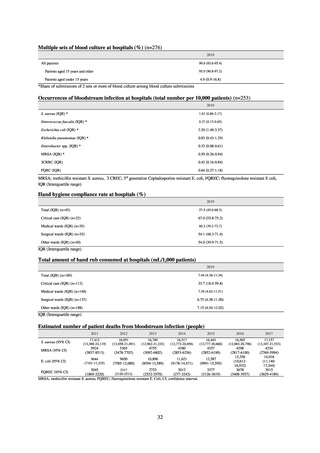
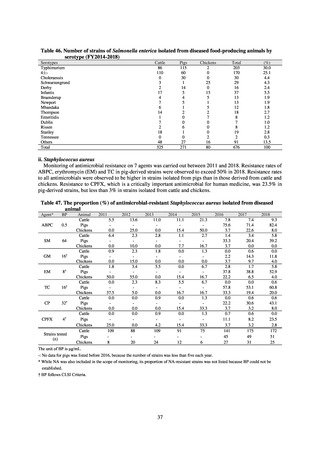
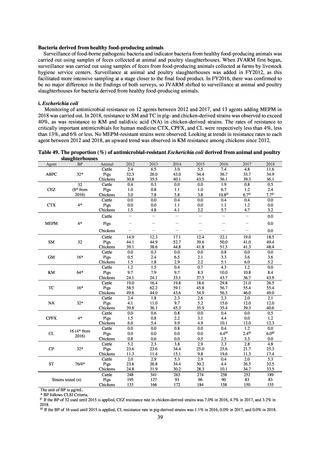
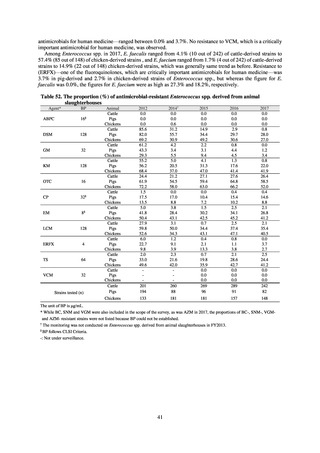
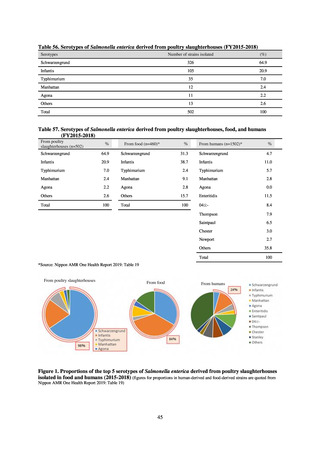
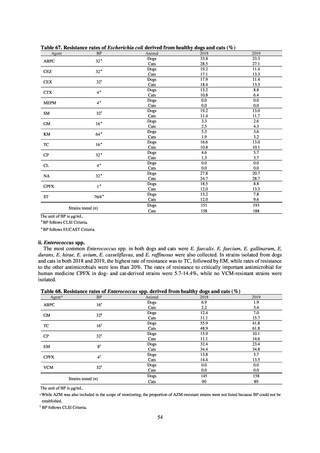
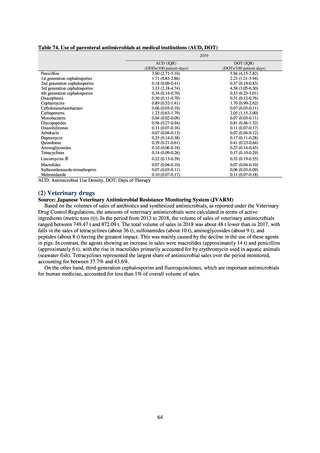
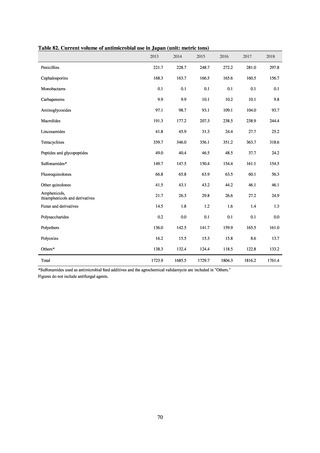
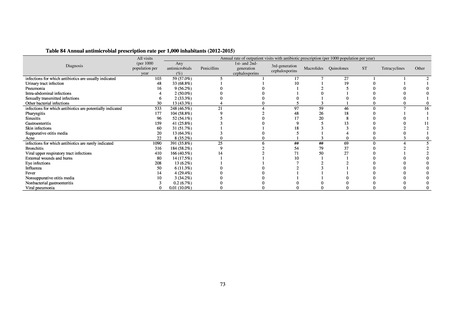
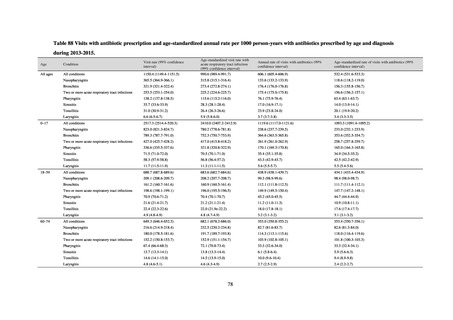
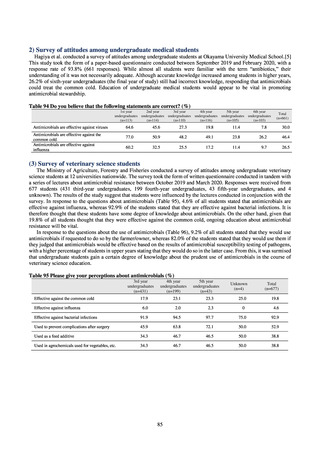
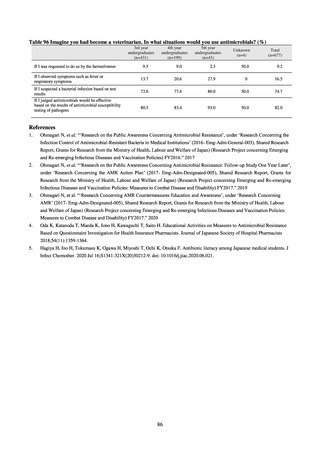
Table 72 shows antimicrobial use based on the AWaRe classification recommended by the WHO as an
indicator of antimicrobial stewardship. Carried in the 20th edition of the WHO Model Lists of Essential Medicines,
the AWaRe classification is an antimicrobial classification system that is applied as an indicator of antimicrobial
stewardship. It classifies antimicrobials into four categories: Access (first- or second-choice antimicrobials used
for treating common infections, regarding whose resistance potential there is little concern, and which should be
made widely available by all countries in high-quality formulations at a reasonable cost. Examples include
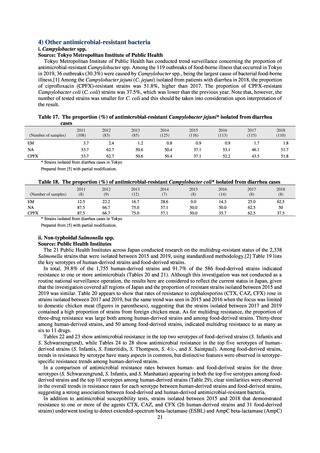
ampicillin and cephalexin), Watch (antimicrobials that should be used only for a limited number of conditions or
applications, as their resistance potential is a source of concern. Examples include vancomycin, meropenem,
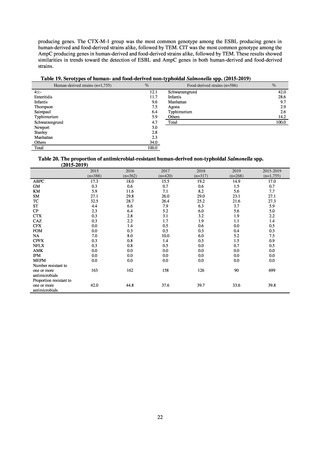
levofloxacin, and ceftriaxone), Reserve (antimicrobials that should be used as the last resort when no other
alternatives can be used. Examples include tigecycline, colistin, and daptomycin), and Unclassified. This
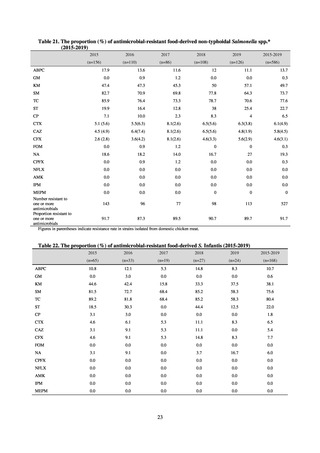
classification was amended in 2019 to add the new category of “discouraged antibiotics,” consisting of
antimicrobials whose clinical use the WHO does not recommend (for example, cefoperazone-sulbactam). The
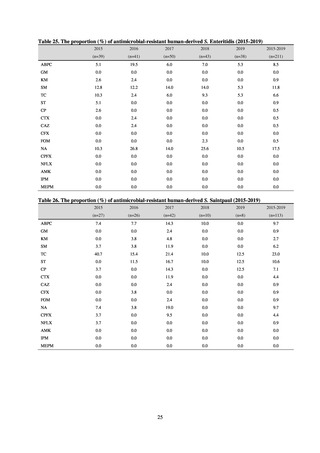
WHO has set a target of at least 60% of antimicrobial consumption being from medicines in the Access Group.
While consumption of antimicrobials in the Access Group as a proportion of total use is lower in Japan than other
countries,[6] the figure has risen gradually over the years from 13.0% to 20.4%, with the percentage of
antimicrobials in the Watch Group falling from 85.5% to 78.3%. Close scrutiny will continue to be required, due
to concerns about the impact of supply shortages and the COVID-19 pandemic.
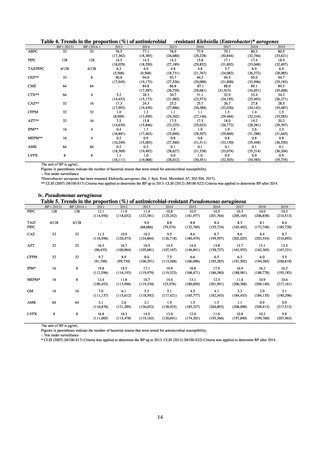
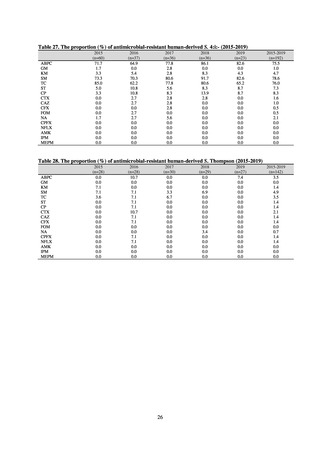
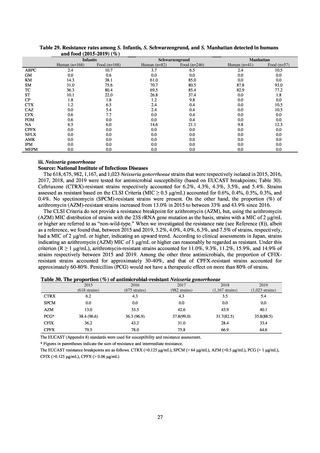
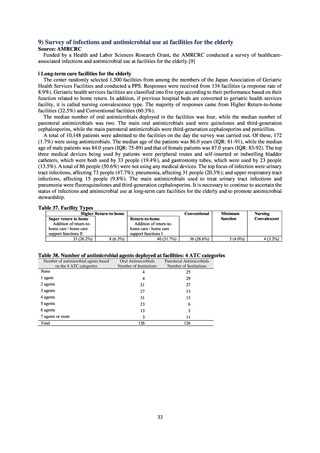
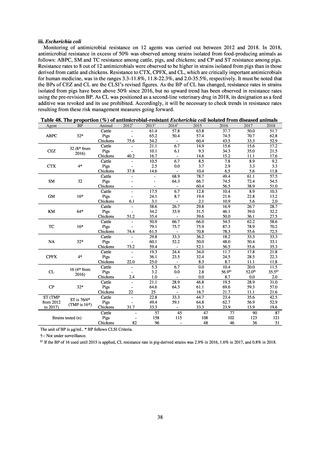
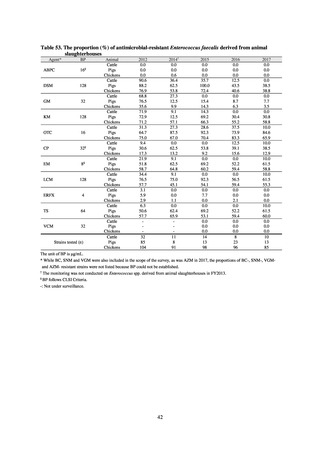
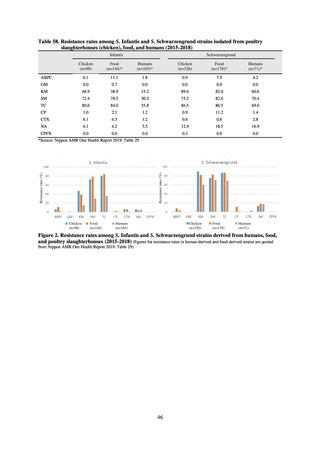
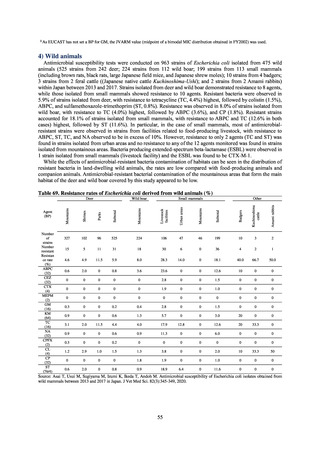
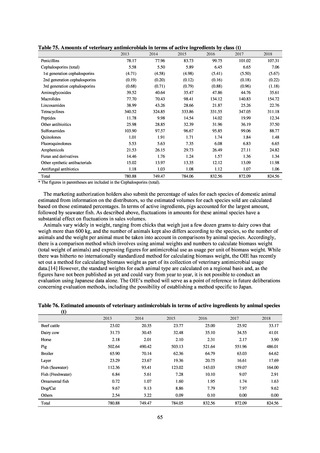
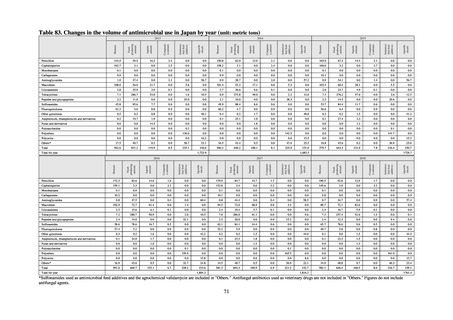
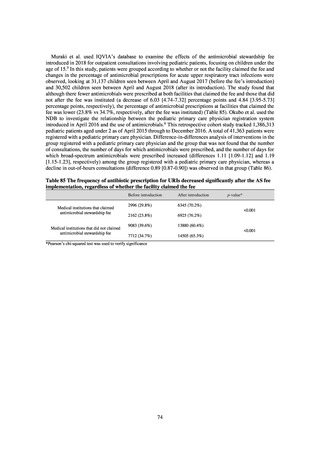
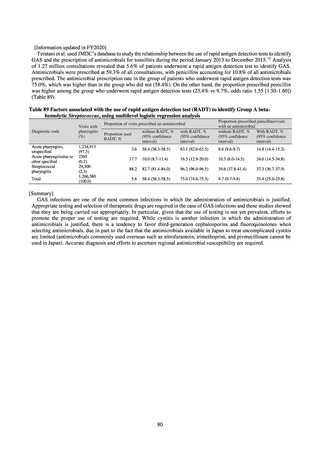
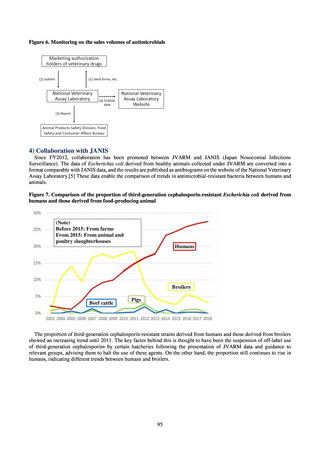
A survey of oral and parenteral antimicrobial use in terms of potency by weight from a One Health perspective
(Table 73) showed no change in overall use. One of the main reasons for the discrepancy between this and the
standardized figures expressed as DID is believed to be the effect of the increased parenteral usage of
ampicillin/sulbactam, which has a high-potency daily dosage and is used to treat aspiration pneumonia in elderly
people.
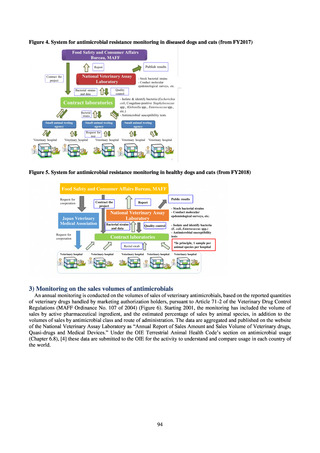
Factors such as the increasing number of elderly people make it difficult to reduce the use of parenteral
antimicrobials in Japan. However, the National Action Plan on AMR has demonstrated some level of effectiveness,
as a fall in oral antimicrobial use was observed after its publication. In addition, a system for continuous monitoring
of antimicrobial use, which was one of the strategies in the National Action Plan on AMR, has been put in place.
While the rate of decline in oral antimicrobial use has slowed, there are limits to the ability to use the volume of
sales to forecast the optimal level of antimicrobial use in patients requiring oral antimicrobials in Japan. Ongoing
efforts to ascertain the extent of antimicrobial use as part of Japan’s measures to combat AMR will continue to be
needed to facilitate evaluation of the selective pressure of antimicrobials. In addition, it will be vital to ascertain
the purpose of antimicrobial use and implement other new strategies enabling the appropriateness of measures to
be assessed.
61
indicator of antimicrobial stewardship. Carried in the 20th edition of the WHO Model Lists of Essential Medicines,
the AWaRe classification is an antimicrobial classification system that is applied as an indicator of antimicrobial
stewardship. It classifies antimicrobials into four categories: Access (first- or second-choice antimicrobials used
for treating common infections, regarding whose resistance potential there is little concern, and which should be
made widely available by all countries in high-quality formulations at a reasonable cost. Examples include
ampicillin and cephalexin), Watch (antimicrobials that should be used only for a limited number of conditions or
applications, as their resistance potential is a source of concern. Examples include vancomycin, meropenem,
levofloxacin, and ceftriaxone), Reserve (antimicrobials that should be used as the last resort when no other
alternatives can be used. Examples include tigecycline, colistin, and daptomycin), and Unclassified. This
classification was amended in 2019 to add the new category of “discouraged antibiotics,” consisting of
antimicrobials whose clinical use the WHO does not recommend (for example, cefoperazone-sulbactam). The
WHO has set a target of at least 60% of antimicrobial consumption being from medicines in the Access Group.
While consumption of antimicrobials in the Access Group as a proportion of total use is lower in Japan than other
countries,[6] the figure has risen gradually over the years from 13.0% to 20.4%, with the percentage of
antimicrobials in the Watch Group falling from 85.5% to 78.3%. Close scrutiny will continue to be required, due
to concerns about the impact of supply shortages and the COVID-19 pandemic.
A survey of oral and parenteral antimicrobial use in terms of potency by weight from a One Health perspective
(Table 73) showed no change in overall use. One of the main reasons for the discrepancy between this and the
standardized figures expressed as DID is believed to be the effect of the increased parenteral usage of
ampicillin/sulbactam, which has a high-potency daily dosage and is used to treat aspiration pneumonia in elderly
people.
Factors such as the increasing number of elderly people make it difficult to reduce the use of parenteral
antimicrobials in Japan. However, the National Action Plan on AMR has demonstrated some level of effectiveness,
as a fall in oral antimicrobial use was observed after its publication. In addition, a system for continuous monitoring
of antimicrobial use, which was one of the strategies in the National Action Plan on AMR, has been put in place.
While the rate of decline in oral antimicrobial use has slowed, there are limits to the ability to use the volume of
sales to forecast the optimal level of antimicrobial use in patients requiring oral antimicrobials in Japan. Ongoing
efforts to ascertain the extent of antimicrobial use as part of Japan’s measures to combat AMR will continue to be
needed to facilitate evaluation of the selective pressure of antimicrobials. In addition, it will be vital to ascertain
the purpose of antimicrobial use and implement other new strategies enabling the appropriateness of measures to
be assessed.
61