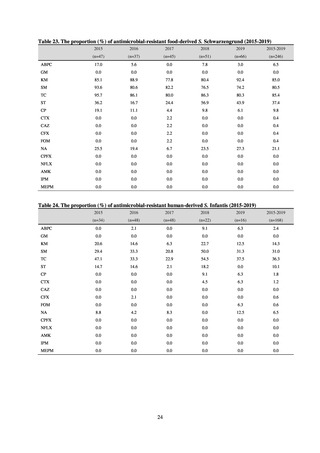
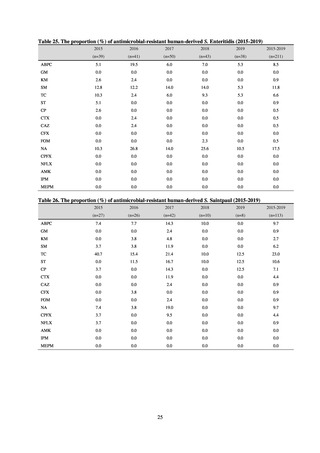
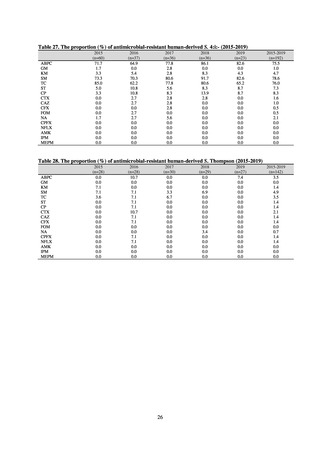
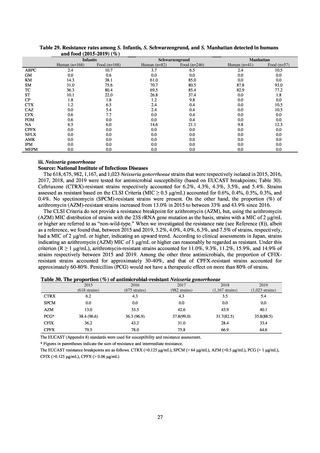
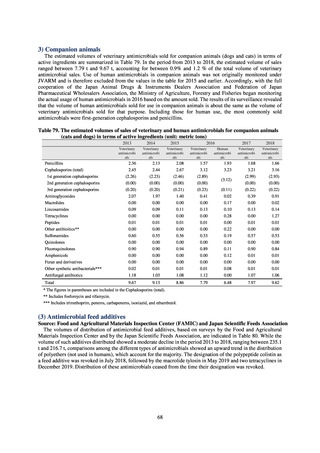
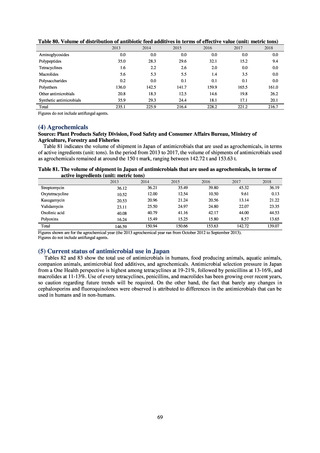
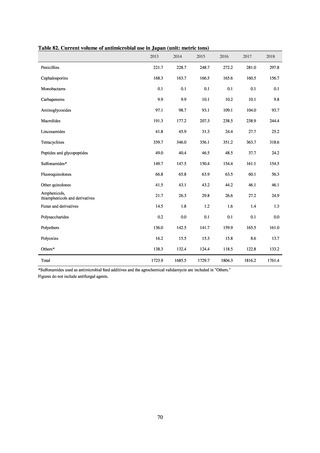
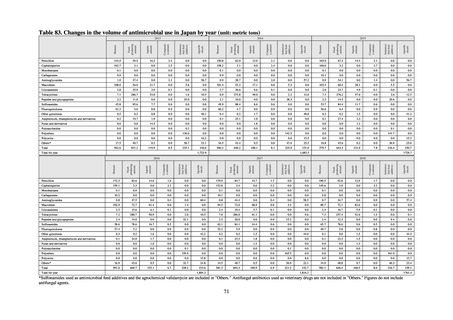
よむ、つかう、まなぶ。
【参考資料3】【英版R4.1.17】Nippon AMR One Health Report (NAOR) 2020 (92 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23261.html |
| 出典情報 | 国際的に脅威となる感染症対策関係閣僚会議 薬剤耐性ワンヘルス動向調査検討会(第9回 1/17)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
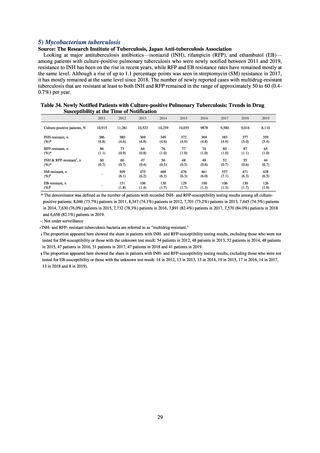
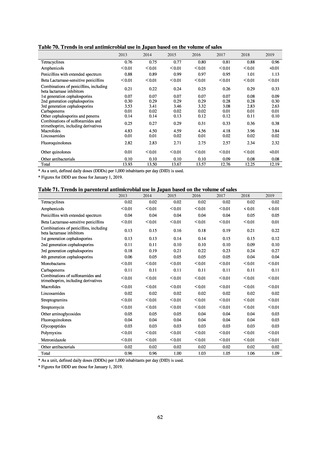
(3) Japan Surveillance for Infection Prevention and Healthcare Epidemiology (J-SIPHE)
1) Overview
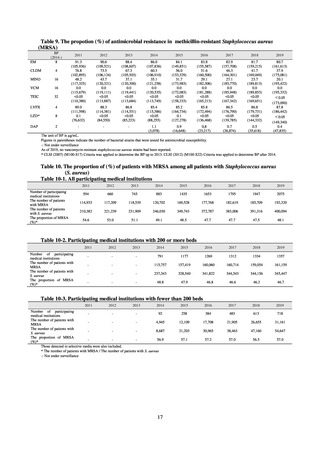
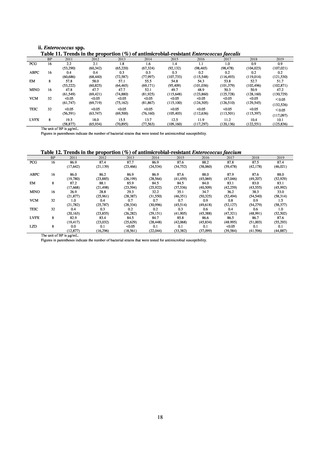
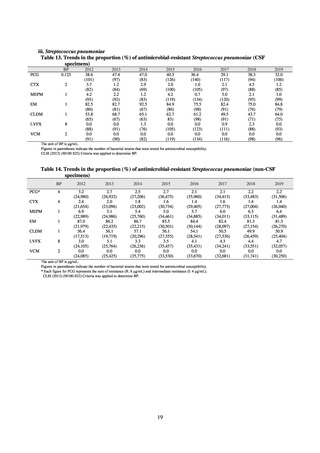
The AMR Clinical Reference Center (AMRCRC) operates the J-SIPHE system, which can be used for AMR measures
at hospitals as well as for promoting regional partnerships. The J-SIPHE 2019 Annual Report covers a total of 581
participating medical institutions (449 calculating Infection Prevention and Control Premium 1, 127 calculating Infection
Prevention and Control Premium 2, and 5 calculating no premium), with the number of participating medical institutions
standing at more than 600 as of October 2020.
The purpose of this system is to collate information for use by participating medical institutions and their local
communities. It covers such information as the treatment status of infectious diseases at participating institutions
nationwide, infection control and antimicrobial stewardship initiatives, the incidence of healthcare-associated infections,
the emergence of major bacteria and antimicrobial-resistant bacteria, the incidence of bloodstream infections by such
bacteria, and antimicrobial use. It also plays a part in developing benchmarks for measures to combat AMR.
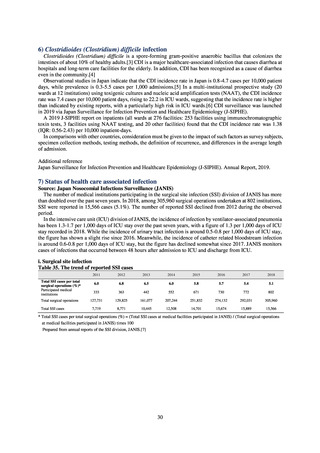
2) System
Participation in this system is based on applications by groups composed of collaborating medical institutions authorized
to treat patients with health insurance coverage in Infection Prevention and Control Premium 1 and Infection Prevention
and Control Premium 2, and institutions not calculating Infection Prevention and Control Premium 1. Participating
institutions may share information within their group based on unified standards, in order to assist in formulating measures
to combat AMR that tap into their networks of community relationships. The system is capable of collating and visualizing
the necessary data concerning measures to combat AMR in a way that minimizes the burden on participating institutions
by making secondary use of existing information such as information fed back to the clinical laboratory division of JANIS
and integrated EF files for admissions.
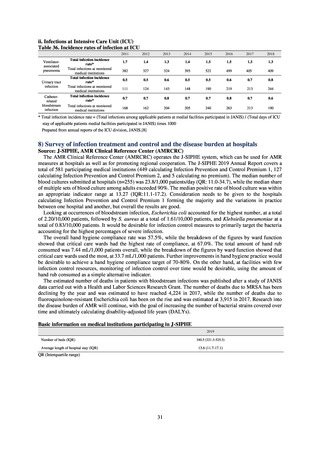
3) Prospects
Most of the institutions participating at present are in the Infection Prevention and Control Premium 1 category, but
improvements to develop a system more conducive to community partnerships are required, to build a system that is more
accessible for institutions in the Infection Prevention and Control Premium 2 and those calculating no premium, so that use
of the system in community cooperation conferences is more meaningful
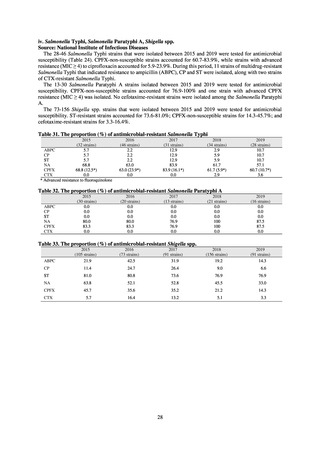
(4) Trend surveillance of antimicrobial-resistant Mycobacterium tuberculosis
1) Overview
registered tuberculosis patient information system is a part of NESID including: new tuberculosis patients and latent
tuberculosis patients who are registered from January 1 to December 31 of a registration year; and all tuberculosis patients
who are registered as of December 31 of the calendar year. In principle, information in this system pertains to tuberculosis
patients, and focuses on the number of incidence case and incidence rate, the number of patients with tuberoses, treatment
status, the number of deaths from tuberculosis, and so on. Information regarding tuberculosis bacillus as the causal bacteria
is limited to the smear positive ratio, the number of culture-positive patients, drug-susceptibility testing data, and so on.
Though limited, this report exclusively provides routine national information regarding antimicrobial-resistant tuberculosis
bacillus.
2) Survey methods
Based on the registered tuberculosis patient information, the results of drug-susceptibility testing in newly registered
patients with culture-positive pulmonary tuberculosis are aggregated. The entry of this information item used to be optional,
before the Ordinance for the Partial Revision of the Enforcement Regulation of the Act on the Prevention of Infectious
Diseases and Medical Care for Patients with Infectious Diseases (MHLW Ordinance No. 101 of 2015, effective May 21,
2015) added "the results of drug-susceptibility testing" under "Conditions of disease" in Item 4, Paragraph 1, Article 27-8.
3) System
When physicians diagnose and report a tuberculosis case to Public Health Center collect, corresponding public health
nurses collect detailed information from patients and physicians. Drug-susceptibility testing data are considered to be
collected mostly from hospital and commercial laboratories. Those individual data are entered by Public Health Centers
across Japan into NESID.
4) Prospects
The surveillance based on the registered tuberculosis patient information system contains the susceptibility results of
newly registered patients with culture-positive pulmonary tuberculosis, as reported from all medical institutions. Therefore,
data are considered nationally representative. Improvement in the entry rate of drug-susceptibility testing results
(approximately 80% at present); the establishment of a system for nationwide quality assurance for drug-susceptibility
testing; and the quality control of data entry are warranted.
91
1) Overview
The AMR Clinical Reference Center (AMRCRC) operates the J-SIPHE system, which can be used for AMR measures
at hospitals as well as for promoting regional partnerships. The J-SIPHE 2019 Annual Report covers a total of 581
participating medical institutions (449 calculating Infection Prevention and Control Premium 1, 127 calculating Infection
Prevention and Control Premium 2, and 5 calculating no premium), with the number of participating medical institutions
standing at more than 600 as of October 2020.
The purpose of this system is to collate information for use by participating medical institutions and their local
communities. It covers such information as the treatment status of infectious diseases at participating institutions
nationwide, infection control and antimicrobial stewardship initiatives, the incidence of healthcare-associated infections,
the emergence of major bacteria and antimicrobial-resistant bacteria, the incidence of bloodstream infections by such
bacteria, and antimicrobial use. It also plays a part in developing benchmarks for measures to combat AMR.
2) System
Participation in this system is based on applications by groups composed of collaborating medical institutions authorized
to treat patients with health insurance coverage in Infection Prevention and Control Premium 1 and Infection Prevention
and Control Premium 2, and institutions not calculating Infection Prevention and Control Premium 1. Participating
institutions may share information within their group based on unified standards, in order to assist in formulating measures
to combat AMR that tap into their networks of community relationships. The system is capable of collating and visualizing
the necessary data concerning measures to combat AMR in a way that minimizes the burden on participating institutions
by making secondary use of existing information such as information fed back to the clinical laboratory division of JANIS
and integrated EF files for admissions.
3) Prospects
Most of the institutions participating at present are in the Infection Prevention and Control Premium 1 category, but
improvements to develop a system more conducive to community partnerships are required, to build a system that is more
accessible for institutions in the Infection Prevention and Control Premium 2 and those calculating no premium, so that use
of the system in community cooperation conferences is more meaningful
(4) Trend surveillance of antimicrobial-resistant Mycobacterium tuberculosis
1) Overview
registered tuberculosis patient information system is a part of NESID including: new tuberculosis patients and latent
tuberculosis patients who are registered from January 1 to December 31 of a registration year; and all tuberculosis patients
who are registered as of December 31 of the calendar year. In principle, information in this system pertains to tuberculosis
patients, and focuses on the number of incidence case and incidence rate, the number of patients with tuberoses, treatment
status, the number of deaths from tuberculosis, and so on. Information regarding tuberculosis bacillus as the causal bacteria
is limited to the smear positive ratio, the number of culture-positive patients, drug-susceptibility testing data, and so on.
Though limited, this report exclusively provides routine national information regarding antimicrobial-resistant tuberculosis
bacillus.
2) Survey methods
Based on the registered tuberculosis patient information, the results of drug-susceptibility testing in newly registered
patients with culture-positive pulmonary tuberculosis are aggregated. The entry of this information item used to be optional,
before the Ordinance for the Partial Revision of the Enforcement Regulation of the Act on the Prevention of Infectious
Diseases and Medical Care for Patients with Infectious Diseases (MHLW Ordinance No. 101 of 2015, effective May 21,
2015) added "the results of drug-susceptibility testing" under "Conditions of disease" in Item 4, Paragraph 1, Article 27-8.
3) System
When physicians diagnose and report a tuberculosis case to Public Health Center collect, corresponding public health
nurses collect detailed information from patients and physicians. Drug-susceptibility testing data are considered to be
collected mostly from hospital and commercial laboratories. Those individual data are entered by Public Health Centers
across Japan into NESID.
4) Prospects
The surveillance based on the registered tuberculosis patient information system contains the susceptibility results of
newly registered patients with culture-positive pulmonary tuberculosis, as reported from all medical institutions. Therefore,
data are considered nationally representative. Improvement in the entry rate of drug-susceptibility testing results
(approximately 80% at present); the establishment of a system for nationwide quality assurance for drug-susceptibility
testing; and the quality control of data entry are warranted.
91