よむ、つかう、まなぶ。
03資料1-1森野委員提出資料(高齢者に対するインフルエンザワクチンファクトシート) (41 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64997.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第32回 10/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
and 2016-2017 influenza vaccination of Medicare beneficiaries. Vaccine 2019; 37(43): 6543-9.
74.
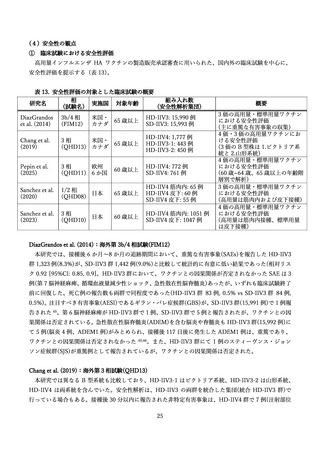
DiazGranados CA, Dunning AJ, Jordanov E, Landolfi V, Denis M, Talbot HK. High-dose
trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity
and relative efficacy during the 2009-2010 season. Vaccine 2013; 31(6): 861-6.
75.
Comber L, E OM, Jordan K, et al. Systematic review of the efficacy, effectiveness and safety
of high-dose seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in
individuals ≥18 years of age. Rev Med Virol 2023; 33(3): e2330.
76.
Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind
controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine
in adults 65 years of age and older. J Infect Dis 2009; 200(2): 172-80.
77.
Caldera F, Mercer M, Samson SI, Pitt JM, Hayney MS. Influenza vaccination in
immunocompromised populations: Strategies to improve immunogenicity. Vaccine 2021; 39 Suppl 1: A15a23.
78.
Chong PP, Handler L, Weber DJ. A Systematic Review of Safety and Immunogenicity of
Influenza Vaccination Strategies in Solid Organ Transplant Recipients. Clin Infect Dis 2018; 66(11):
1802-11.
79.
Izikson R, Brune D, Bolduc JS, et al. Safety and immunogenicity of a high-dose quadrivalent
influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine
in adults aged ≥65 years: a phase 2, randomised, open-label study. Lancet Respir Med 2022; 10(4): 392402.
80.
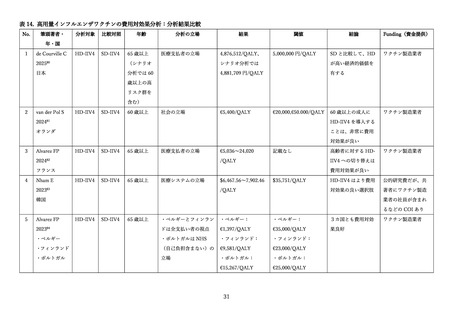
de Courville C, Tadera C, Arashiro T, et al. Cost-effectiveness and public health impact of using
high dose influenza vaccine in the Japanese older adults. J Med Econ 2025; 28(1): 544-55.
81.
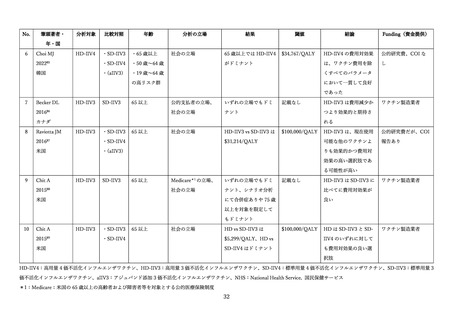
van der Pol S, Zeevat F, Postma MJ, Boersma C. Cost-effectiveness of high-dose influenza
vaccination in the Netherlands: Incorporating the impact on both respiratory and cardiovascular
hospitalizations. Vaccine 2024; 42(15): 3429-36.
82.
Alvarez FP, Allard L, Bianic F, et al. Cost-effectiveness and public health impact of using high
dose quadrivalent influenza vaccine in the French older adults population. J Med Econ 2024; 27(1): 13007.
83.
Nham E, Seong H, Hyun H, et al. Cost-effectiveness of high-dose quadrivalent influenza vaccine
versus standard-dose quadrivalent influenza vaccine for older people in a country with high influenza
vaccination rate. Hum Vaccin Immunother 2023; 19(3): 2266233.
84.
Alvarez FP, Chevalier P, Borms M, et al. Cost-effectiveness of influenza vaccination with a high
dose quadrivalent vaccine of the elderly population in Belgium, Finland, and Portugal. J Med Econ 2023;
26(1): 710-9.
85.
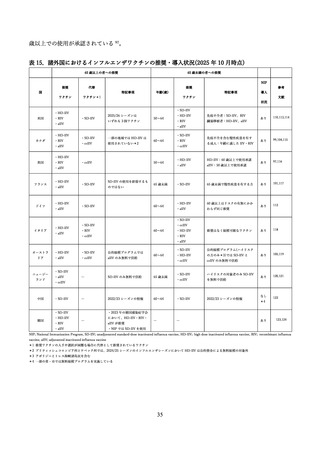
Choi MJ, Shin G, Kang D, et al. Cost-Effectiveness of Influenza Vaccination Strategies in
Adults: Older Adults Aged ≥65 Years, Adults Aged 50-64 Years, and At-Risk Adults Aged 19-64 Years.
Vaccines (Basel) 2022; 10(3).
86.
Becker DL, Chit A, DiazGranados CA, Maschio M, Yau E, Drummond M. High-dose inactivated
41
74.
DiazGranados CA, Dunning AJ, Jordanov E, Landolfi V, Denis M, Talbot HK. High-dose
trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity
and relative efficacy during the 2009-2010 season. Vaccine 2013; 31(6): 861-6.
75.
Comber L, E OM, Jordan K, et al. Systematic review of the efficacy, effectiveness and safety
of high-dose seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in
individuals ≥18 years of age. Rev Med Virol 2023; 33(3): e2330.
76.
Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind
controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine
in adults 65 years of age and older. J Infect Dis 2009; 200(2): 172-80.
77.
Caldera F, Mercer M, Samson SI, Pitt JM, Hayney MS. Influenza vaccination in
immunocompromised populations: Strategies to improve immunogenicity. Vaccine 2021; 39 Suppl 1: A15a23.
78.
Chong PP, Handler L, Weber DJ. A Systematic Review of Safety and Immunogenicity of
Influenza Vaccination Strategies in Solid Organ Transplant Recipients. Clin Infect Dis 2018; 66(11):
1802-11.
79.
Izikson R, Brune D, Bolduc JS, et al. Safety and immunogenicity of a high-dose quadrivalent
influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine
in adults aged ≥65 years: a phase 2, randomised, open-label study. Lancet Respir Med 2022; 10(4): 392402.
80.
de Courville C, Tadera C, Arashiro T, et al. Cost-effectiveness and public health impact of using
high dose influenza vaccine in the Japanese older adults. J Med Econ 2025; 28(1): 544-55.
81.
van der Pol S, Zeevat F, Postma MJ, Boersma C. Cost-effectiveness of high-dose influenza
vaccination in the Netherlands: Incorporating the impact on both respiratory and cardiovascular
hospitalizations. Vaccine 2024; 42(15): 3429-36.
82.
Alvarez FP, Allard L, Bianic F, et al. Cost-effectiveness and public health impact of using high
dose quadrivalent influenza vaccine in the French older adults population. J Med Econ 2024; 27(1): 13007.
83.
Nham E, Seong H, Hyun H, et al. Cost-effectiveness of high-dose quadrivalent influenza vaccine
versus standard-dose quadrivalent influenza vaccine for older people in a country with high influenza
vaccination rate. Hum Vaccin Immunother 2023; 19(3): 2266233.
84.
Alvarez FP, Chevalier P, Borms M, et al. Cost-effectiveness of influenza vaccination with a high
dose quadrivalent vaccine of the elderly population in Belgium, Finland, and Portugal. J Med Econ 2023;
26(1): 710-9.
85.
Choi MJ, Shin G, Kang D, et al. Cost-Effectiveness of Influenza Vaccination Strategies in
Adults: Older Adults Aged ≥65 Years, Adults Aged 50-64 Years, and At-Risk Adults Aged 19-64 Years.
Vaccines (Basel) 2022; 10(3).
86.
Becker DL, Chit A, DiazGranados CA, Maschio M, Yau E, Drummond M. High-dose inactivated
41