よむ、つかう、まなぶ。
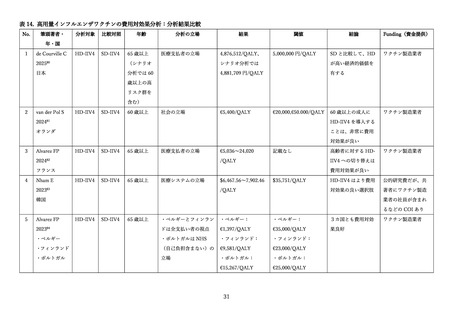
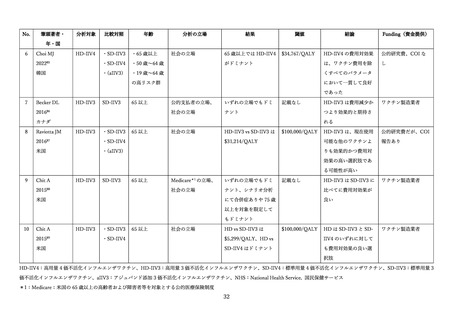
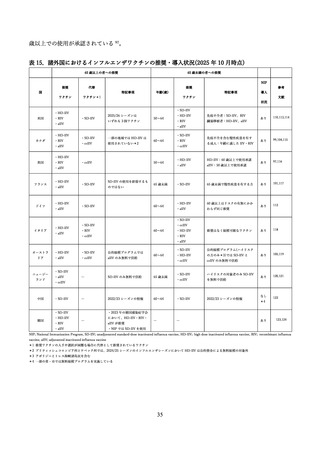
03資料1-1森野委員提出資料(高齢者に対するインフルエンザワクチンファクトシート) (38 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_64997.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第32回 10/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
32.
国立感染症研究所. 令和 7 年度インフルエンザワクチン用製造株とその推奨理由. 2025.
https://www.mhlw.go.jp/content/10906000/001494611.pdf.
33.
一般財団法人阪大微生物病研究会. インフルエンザ HA ワクチン「ビケン HA」. 2024.
https://www.info.pmda.go.jp/go/pack/631340FA1047_1_35/?view=frame&style=XML&lang=ja (accessed
2025/07/03
34.
KM バイオロジクス株式会社. インフルエンザ HA ワクチン「KMB」. 2024.
https://www.info.pmda.go.jp/go/pack/631340FA1101_7_10/?view=frame&style=XML&lang=ja
35.
デンカ株式会社. インフルエンザ HA ワクチン「生研」. 2024.
https://www.info.pmda.go.jp/go/pack/631340FA1055_5_07/?view=frame&style=XML&lang=ja
36.
Treanor JJ. CLINICAL PRACTICE. Influenza Vaccination. N Engl J Med 2016; 375(13): 1261-8.
37.
Bresee JS, Fry, A. M., Sambhara, S., Cox, N. J. Inactivated Influenza Vaccines.
Vaccines. 7th
ed. Philadelphia: Saunders; 2018: 456-88.
38.
サノフィ株式会社. エフルエルダ筋注. 2024.
https://www.info.pmda.go.jp/go/pack/631341RG1020_1_01/?view=frame&style=XML&lang=ja (accessed
2025/07/03)
39.
Sanchez L, Matsuoka O, Inoue S, et al. Immunogenicity and safety of high-dose quadrivalent
influenza vaccine in Japanese adults >/=65 years of age: a randomized controlled clinical trial. Hum
Vaccin Immunother 2020; 16(4): 858-66.
40.
Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-
inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg
(Lond) 1972; 70(4): 767-77.
41.
Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull
1979; 35(1): 69-75.
42.
Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a
quantitative review. Vaccine 2006; 24(8): 1159-69.
43.
DiazGranados CA, Saway W, Gouaux J, et al. Safety and immunogenicity of high-dose trivalent
inactivated influenza vaccine in adults 50-64 years of age. Vaccine 2015; 33(51): 7188-93.
44.
Volling C, Coleman BL, Katz K, et al. Immunogenicity and reactogenicity of high- vs. standard-
dose trivalent inactivated influenza vaccine in healthcare workers: a pilot randomized controlled trial.
Clin Microbiol Infect 2019; 25(2): 217-24.
45.
Sanchez L, Nakama T, Nagai H, et al. Superior immunogenicity of high-dose quadrivalent
inactivated influenza vaccine versus Standard-Dose vaccine in Japanese Adults ≥ 60 years of age: Results
from a phase III, randomized clinical trial. Vaccine 2023; 41(15): 2553-61.
46.
Loeb N, Andrew MK, Loeb M, et al. Frailty Is Associated With Increased Hemagglutination-
Inhibition Titers in a 4-Year Randomized Trial Comparing Standard- and High-Dose Influenza
Vaccination. Open Forum Infect Dis 2020; 7(5): ofaa148.
47.
Strowd RE, Russell G, Hsu FC, et al. Immunogenicity of high-dose influenza vaccination in
patients with primary central nervous system malignancy. Neurooncol Pract 2018; 5(3): 176-83.
38
国立感染症研究所. 令和 7 年度インフルエンザワクチン用製造株とその推奨理由. 2025.
https://www.mhlw.go.jp/content/10906000/001494611.pdf.
33.
一般財団法人阪大微生物病研究会. インフルエンザ HA ワクチン「ビケン HA」. 2024.
https://www.info.pmda.go.jp/go/pack/631340FA1047_1_35/?view=frame&style=XML&lang=ja (accessed
2025/07/03
34.
KM バイオロジクス株式会社. インフルエンザ HA ワクチン「KMB」. 2024.
https://www.info.pmda.go.jp/go/pack/631340FA1101_7_10/?view=frame&style=XML&lang=ja
35.
デンカ株式会社. インフルエンザ HA ワクチン「生研」. 2024.
https://www.info.pmda.go.jp/go/pack/631340FA1055_5_07/?view=frame&style=XML&lang=ja
36.
Treanor JJ. CLINICAL PRACTICE. Influenza Vaccination. N Engl J Med 2016; 375(13): 1261-8.
37.
Bresee JS, Fry, A. M., Sambhara, S., Cox, N. J. Inactivated Influenza Vaccines.
Vaccines. 7th
ed. Philadelphia: Saunders; 2018: 456-88.
38.
サノフィ株式会社. エフルエルダ筋注. 2024.
https://www.info.pmda.go.jp/go/pack/631341RG1020_1_01/?view=frame&style=XML&lang=ja (accessed
2025/07/03)
39.
Sanchez L, Matsuoka O, Inoue S, et al. Immunogenicity and safety of high-dose quadrivalent
influenza vaccine in Japanese adults >/=65 years of age: a randomized controlled clinical trial. Hum
Vaccin Immunother 2020; 16(4): 858-66.
40.
Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-
inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg
(Lond) 1972; 70(4): 767-77.
41.
Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull
1979; 35(1): 69-75.
42.
Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a
quantitative review. Vaccine 2006; 24(8): 1159-69.
43.
DiazGranados CA, Saway W, Gouaux J, et al. Safety and immunogenicity of high-dose trivalent
inactivated influenza vaccine in adults 50-64 years of age. Vaccine 2015; 33(51): 7188-93.
44.
Volling C, Coleman BL, Katz K, et al. Immunogenicity and reactogenicity of high- vs. standard-
dose trivalent inactivated influenza vaccine in healthcare workers: a pilot randomized controlled trial.
Clin Microbiol Infect 2019; 25(2): 217-24.
45.
Sanchez L, Nakama T, Nagai H, et al. Superior immunogenicity of high-dose quadrivalent
inactivated influenza vaccine versus Standard-Dose vaccine in Japanese Adults ≥ 60 years of age: Results
from a phase III, randomized clinical trial. Vaccine 2023; 41(15): 2553-61.
46.
Loeb N, Andrew MK, Loeb M, et al. Frailty Is Associated With Increased Hemagglutination-
Inhibition Titers in a 4-Year Randomized Trial Comparing Standard- and High-Dose Influenza
Vaccination. Open Forum Infect Dis 2020; 7(5): ofaa148.
47.
Strowd RE, Russell G, Hsu FC, et al. Immunogenicity of high-dose influenza vaccination in
patients with primary central nervous system malignancy. Neurooncol Pract 2018; 5(3): 176-83.
38