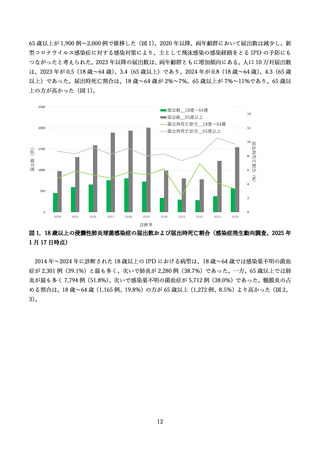
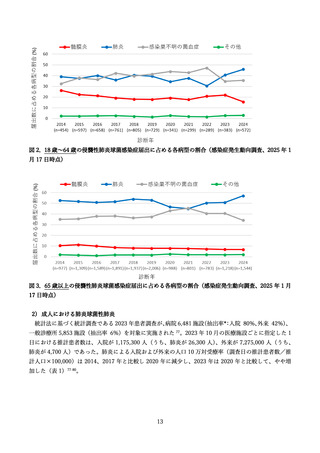
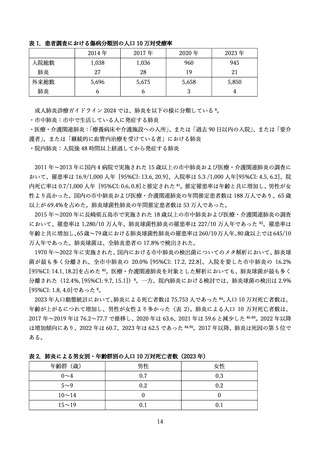
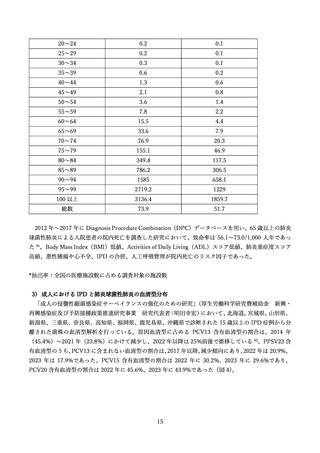
よむ、つかう、まなぶ。
10参考資料1-2 成人用肺炎球菌ワクチンファクトシート[4.9MB] (46 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_70339.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会(第64回 2/12)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=25&SelectedCountryIdByDisease=-1
(accessed 2024/12/18).
182.
Services HNZHIa. Pneumococcal vaccine. 2024/7/9. https://info.health.nz/immunisations/vaccines-
aotearoa/pneumococcal-vaccine (accessed 2025/4/4).
183.
Guo X, Li J, Qiu J, et al. Persistence of antibody to 23-valent pneumococcal polysaccharide vaccine: a
5-year prospective follow-up cohort study. Expert Rev Vaccines 2024; 23(1): 237-45.
184.
Center for Disease Control and Prevention. Summary of Risk-based Pneumococcal Vaccination
Recommendations. 2024/10/26 2024. https://www.cdc.gov/pneumococcal/hcp/vaccinerecommendations/risk-indications.html (accessed 2025/4/4).
185.
Government of Canada. Pneumococcal vaccines: Canadian Immunization Guide. 2024/11/15 2024.
https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guidepart-4-active-vaccines/page-16-pneumococcal-vaccine.html (accessed 2025/4/4).
186.
UK Health Security Agency. Green book Chapter 25: Pneumococcal. 2023/7/23 2023.
https://assets.publishing.service.gov.uk/media/64d68d6edd15ff000d278019/Green_Book_Chapter_25_Pneu
mococcal_27_7_23.pdf (accessed 2025/4/4).
187.
Haute Autorite de Sante. Vaccination strategy against pneumococcal infections - Place of the
pneumococcal polysaccharide conjugate vaccine (20-valent, adsorbed) in adults (in French; 機械翻訳).
2023/7/27 2023. https://www.has-sante.fr/jcms/p_3457419/fr/strategie-de-vaccination-contre-les-infectionsa-pneumocoque-place-du-vaccin-pneumococcique-polyosidique-conjugue-20-valent-adsorbe-chez-l-adulte
(accessed 2025/4/4).
188.
Robert Koch Institut. Epidemiologisches Bulletin. 2023/9/28 2023.
https://www.rki.de/DE/Aktuelles/Publikationen/EpidemiologischesBulletin/2023/39_23.pdf?__blob=publicationFile&v=5 (accessed 2025/4/4).
189.
Chinese Center for Disease Control and Prevention. Childhood Immunization Schedule for National
Immunization Program Vaccines — China (Version 2021). 2022/3/2 2022.
https://en.chinacdc.cn/health_topics/immunization/202203/t20220302_257317.html (accessed 2025/4/4).
190.
Wang CP, Lin YT, Du YZ, et al. Impact of innovative immunization strategy on PCV13 vaccination
coverage among children under 5 years in Weifang city, China: A retrospective study. Vaccine 2024; 42(5):
1136-44.
191.
Choi WS, Song JY, Kwon KT, et al. Recommendations for Adult Immunization by the Korean Society
of Infectious Diseases, 2023: Minor Revisions to the 3rd Edition. Infect Chemother 2024; 56(2): 188-203.
192.
Choi WS. Adult Immunization Policy in Korea. Infect Chemother 2023; 55(3): 317-21.
193.
Kang DW, June Choe Y, Lee JY, et al. Cost-effectiveness analysis of the 20-valent pneumococcal
conjugate vaccine for the pediatric population in South Korea. Vaccine 2024; 42(22): 126000.
194.
The Joint Committee on Vaccination and Immunisation. Minute 2023 06. 2023.
https://app.box.com/s/iddfb4ppwkmtjusir2tc (accessed 2025/4/4).
195.
The Joint Committee on Vaccination and Immunisation. Minute of the JCVI Pneumococcal Sub-
committee meeting, 4 April 2023. 2023. https://app.box.com/s/1mrhw4tnughfvbbvujt5thww5f0mjhpv
46
(accessed 2024/12/18).
182.
Services HNZHIa. Pneumococcal vaccine. 2024/7/9. https://info.health.nz/immunisations/vaccines-
aotearoa/pneumococcal-vaccine (accessed 2025/4/4).
183.
Guo X, Li J, Qiu J, et al. Persistence of antibody to 23-valent pneumococcal polysaccharide vaccine: a
5-year prospective follow-up cohort study. Expert Rev Vaccines 2024; 23(1): 237-45.
184.
Center for Disease Control and Prevention. Summary of Risk-based Pneumococcal Vaccination
Recommendations. 2024/10/26 2024. https://www.cdc.gov/pneumococcal/hcp/vaccinerecommendations/risk-indications.html (accessed 2025/4/4).
185.
Government of Canada. Pneumococcal vaccines: Canadian Immunization Guide. 2024/11/15 2024.
https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guidepart-4-active-vaccines/page-16-pneumococcal-vaccine.html (accessed 2025/4/4).
186.
UK Health Security Agency. Green book Chapter 25: Pneumococcal. 2023/7/23 2023.
https://assets.publishing.service.gov.uk/media/64d68d6edd15ff000d278019/Green_Book_Chapter_25_Pneu
mococcal_27_7_23.pdf (accessed 2025/4/4).
187.
Haute Autorite de Sante. Vaccination strategy against pneumococcal infections - Place of the
pneumococcal polysaccharide conjugate vaccine (20-valent, adsorbed) in adults (in French; 機械翻訳).
2023/7/27 2023. https://www.has-sante.fr/jcms/p_3457419/fr/strategie-de-vaccination-contre-les-infectionsa-pneumocoque-place-du-vaccin-pneumococcique-polyosidique-conjugue-20-valent-adsorbe-chez-l-adulte
(accessed 2025/4/4).
188.
Robert Koch Institut. Epidemiologisches Bulletin. 2023/9/28 2023.
https://www.rki.de/DE/Aktuelles/Publikationen/EpidemiologischesBulletin/2023/39_23.pdf?__blob=publicationFile&v=5 (accessed 2025/4/4).
189.
Chinese Center for Disease Control and Prevention. Childhood Immunization Schedule for National
Immunization Program Vaccines — China (Version 2021). 2022/3/2 2022.
https://en.chinacdc.cn/health_topics/immunization/202203/t20220302_257317.html (accessed 2025/4/4).
190.
Wang CP, Lin YT, Du YZ, et al. Impact of innovative immunization strategy on PCV13 vaccination
coverage among children under 5 years in Weifang city, China: A retrospective study. Vaccine 2024; 42(5):
1136-44.
191.
Choi WS, Song JY, Kwon KT, et al. Recommendations for Adult Immunization by the Korean Society
of Infectious Diseases, 2023: Minor Revisions to the 3rd Edition. Infect Chemother 2024; 56(2): 188-203.
192.
Choi WS. Adult Immunization Policy in Korea. Infect Chemother 2023; 55(3): 317-21.
193.
Kang DW, June Choe Y, Lee JY, et al. Cost-effectiveness analysis of the 20-valent pneumococcal
conjugate vaccine for the pediatric population in South Korea. Vaccine 2024; 42(22): 126000.
194.
The Joint Committee on Vaccination and Immunisation. Minute 2023 06. 2023.
https://app.box.com/s/iddfb4ppwkmtjusir2tc (accessed 2025/4/4).
195.
The Joint Committee on Vaccination and Immunisation. Minute of the JCVI Pneumococcal Sub-
committee meeting, 4 April 2023. 2023. https://app.box.com/s/1mrhw4tnughfvbbvujt5thww5f0mjhpv
46