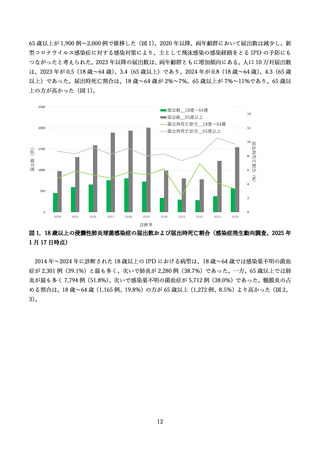
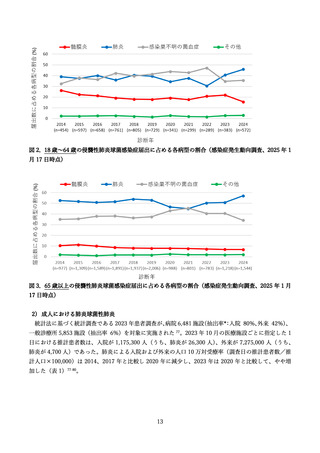
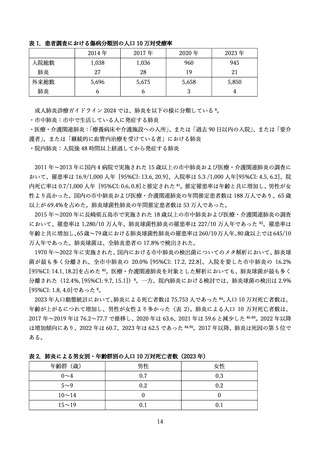
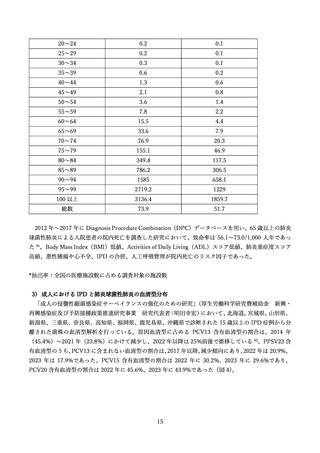
よむ、つかう、まなぶ。
10参考資料1-2 成人用肺炎球菌ワクチンファクトシート[4.9MB] (43 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_70339.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会(第64回 2/12)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
pneumonia in adults. The New England journal of medicine 2015; 372(12): 1114-25.
140.
Lewnard JA, Hong V, Bruxvoort KJ, et al. Burden of Lower Respiratory Tract Infections Preventable
by Adult Immunization With 15- and 20-Valent Pneumococcal Conjugate Vaccines in the United States. Clin
Infect Dis 2023; 77(9): 1340-52.
141.
Heo JY, Seo YB, Choi WS, et al. Effectiveness of Pneumococcal Vaccination Against Pneumococcal
Pneumonia Hospitalization in Older Adults: A Prospective, Test-Negative Study. J Infect Dis 2022; 225(5):
836-45.
142.
Andrews NJ, Waight PA, George RC, Slack MPE, Miller E. Impact and effectiveness of 23-valent
pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and
Wales. Vaccine 2012; 30(48): 6802-8.
143.
Lawrence H, Pick H, Baskaran V, et al. Effectiveness of the 23-valent pneumococcal polysaccharide
vaccine against vaccine serotype pneumococcal pneumonia in adults: A case-control test-negative design study.
PLoS Med 2020; 17(10): e1003326.
144.
van Werkhoven CH, Huijts SM, Bolkenbaas M, Grobbee DE, Bonten MJ. The Impact of Age on the
Efficacy of 13-valent Pneumococcal Conjugate Vaccine in Elderly. Clin Infect Dis 2015; 61(12): 1835-8.
145.
Patterson S, Webber C, Patton M, et al. A post hoc assessment of duration of protection in CAPiTA
(Community Acquired Pneumonia immunization Trial in Adults). Trials in Vaccinology 2016; 5: 92-6.
146.
Thompson A, Lamberth E, Severs J, et al. Phase 1 trial of a 20-valent pneumococcal conjugate
vaccine in healthy adults. Vaccine 2019; 37(42): 6201-7.
147.
Oliveira M, Marquez P, Ennulat C, et al. Post-licensure Safety Surveillance of 20-Valent
Pneumococcal Conjugate Vaccine (PCV20) Among US Adults in the Vaccine Adverse Event Reporting System
(VAERS). Drug Saf 2024.
148.
Tseng HF, Sy LS, Qian L, et al. Pneumococcal Conjugate Vaccine Safety in Elderly Adults. Open
Forum Infect Dis 2018; 5(6): ofy100.
149.
Kobayashi M, Leidner AJ, Gierke R, et al. Expanded Recommendations for Use of Pneumococcal
Conjugate Vaccines Among Adults Aged ≥50 Years: Recommendations of the Advisory Committee on
Immunization Practices - United States, 2024. MMWR Morb Mortal Wkly Rep 2025; 74(1): 1-8.
150.
Sato S, Katsuta T, Kawazoe Y, et al. Immune thrombocytopenic purpura and Guillain-Barré
syndrome after 23-valent pneumococcal polysaccharide vaccination in Japan: The vaccine effectiveness,
networking, and universal safety (VENUS) study. Vaccine 2024; 42(1): 4-7.
151.
Fitz-Patrick D, Young M, Jr., Scott DA, et al. A randomized phase 1 study of the safety and
immunogenicity of 2 novel pneumococcal conjugate vaccines in healthy Japanese adults in the United States.
Hum Vaccin Immunother 2021; 17(7): 2249-56.
152.
Fitz-Patrick D, Young M, Yacisin K, et al. Randomized trial to evaluate the safety, tolerability, and
immunogenicity of a booster (third dose) of BNT162b2 COVID-19 vaccine coadministered with 20-valent
pneumococcal conjugate vaccine in adults ≥65 years old. Vaccine 2023; 41(28): 4190-8.
153.
Bai S, Zhou S, Zhang J, et al. Immunogenicity and safety of different combinations involving a third
booster dose of SARS-CoV-2 inactivated vaccine, inactivated quadrivalent influenza vaccine, and 23-valent
43
140.
Lewnard JA, Hong V, Bruxvoort KJ, et al. Burden of Lower Respiratory Tract Infections Preventable
by Adult Immunization With 15- and 20-Valent Pneumococcal Conjugate Vaccines in the United States. Clin
Infect Dis 2023; 77(9): 1340-52.
141.
Heo JY, Seo YB, Choi WS, et al. Effectiveness of Pneumococcal Vaccination Against Pneumococcal
Pneumonia Hospitalization in Older Adults: A Prospective, Test-Negative Study. J Infect Dis 2022; 225(5):
836-45.
142.
Andrews NJ, Waight PA, George RC, Slack MPE, Miller E. Impact and effectiveness of 23-valent
pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and
Wales. Vaccine 2012; 30(48): 6802-8.
143.
Lawrence H, Pick H, Baskaran V, et al. Effectiveness of the 23-valent pneumococcal polysaccharide
vaccine against vaccine serotype pneumococcal pneumonia in adults: A case-control test-negative design study.
PLoS Med 2020; 17(10): e1003326.
144.
van Werkhoven CH, Huijts SM, Bolkenbaas M, Grobbee DE, Bonten MJ. The Impact of Age on the
Efficacy of 13-valent Pneumococcal Conjugate Vaccine in Elderly. Clin Infect Dis 2015; 61(12): 1835-8.
145.
Patterson S, Webber C, Patton M, et al. A post hoc assessment of duration of protection in CAPiTA
(Community Acquired Pneumonia immunization Trial in Adults). Trials in Vaccinology 2016; 5: 92-6.
146.
Thompson A, Lamberth E, Severs J, et al. Phase 1 trial of a 20-valent pneumococcal conjugate
vaccine in healthy adults. Vaccine 2019; 37(42): 6201-7.
147.
Oliveira M, Marquez P, Ennulat C, et al. Post-licensure Safety Surveillance of 20-Valent
Pneumococcal Conjugate Vaccine (PCV20) Among US Adults in the Vaccine Adverse Event Reporting System
(VAERS). Drug Saf 2024.
148.
Tseng HF, Sy LS, Qian L, et al. Pneumococcal Conjugate Vaccine Safety in Elderly Adults. Open
Forum Infect Dis 2018; 5(6): ofy100.
149.
Kobayashi M, Leidner AJ, Gierke R, et al. Expanded Recommendations for Use of Pneumococcal
Conjugate Vaccines Among Adults Aged ≥50 Years: Recommendations of the Advisory Committee on
Immunization Practices - United States, 2024. MMWR Morb Mortal Wkly Rep 2025; 74(1): 1-8.
150.
Sato S, Katsuta T, Kawazoe Y, et al. Immune thrombocytopenic purpura and Guillain-Barré
syndrome after 23-valent pneumococcal polysaccharide vaccination in Japan: The vaccine effectiveness,
networking, and universal safety (VENUS) study. Vaccine 2024; 42(1): 4-7.
151.
Fitz-Patrick D, Young M, Jr., Scott DA, et al. A randomized phase 1 study of the safety and
immunogenicity of 2 novel pneumococcal conjugate vaccines in healthy Japanese adults in the United States.
Hum Vaccin Immunother 2021; 17(7): 2249-56.
152.
Fitz-Patrick D, Young M, Yacisin K, et al. Randomized trial to evaluate the safety, tolerability, and
immunogenicity of a booster (third dose) of BNT162b2 COVID-19 vaccine coadministered with 20-valent
pneumococcal conjugate vaccine in adults ≥65 years old. Vaccine 2023; 41(28): 4190-8.
153.
Bai S, Zhou S, Zhang J, et al. Immunogenicity and safety of different combinations involving a third
booster dose of SARS-CoV-2 inactivated vaccine, inactivated quadrivalent influenza vaccine, and 23-valent
43