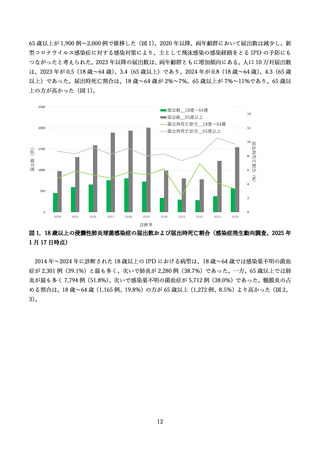
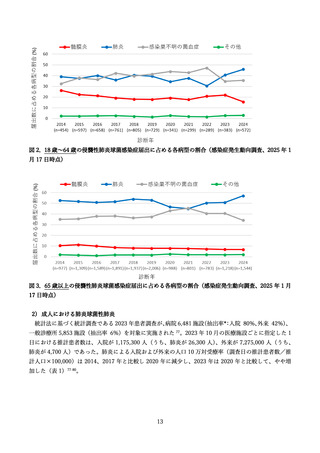
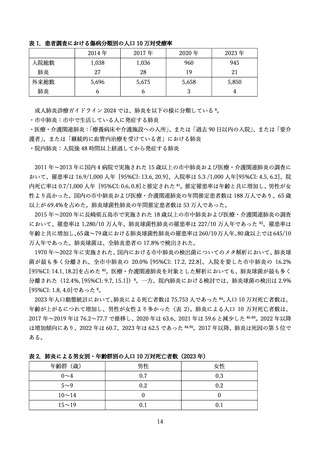
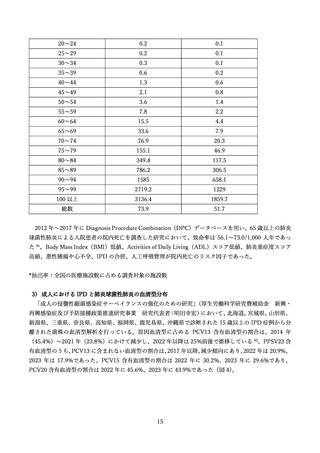
よむ、つかう、まなぶ。
10参考資料1-2 成人用肺炎球菌ワクチンファクトシート[4.9MB] (44 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_70339.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会(第64回 2/12)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
pneumococcal polysaccharide vaccine in adults aged ≥60 years: a phase 4, randomized, open-label study. Front
Immunol 2024; 15: 1437267.
154.
Severance R, Schwartz H, Dagan R, et al. Safety, tolerability, and immunogenicity of V114, a 15-
valent pneumococcal conjugate vaccine, administered concomitantly with influenza vaccine in healthy adults
aged ≥50 years: a randomized phase 3 trial (PNEU-FLU). Hum Vaccin Immunother 2022; 18(1): 1-14.
155.
Cannon K, Cardona JF, Yacisin K, et al. Safety and immunogenicity of a 20-valent pneumococcal
conjugate vaccine coadministered with quadrivalent influenza vaccine: A phase 3 randomized trial. Vaccine
2023; 41(13): 2137-46.
156.
Zhu Z, Sun J, Xie Y, et al. Immunogenicity and Safety of an Inactivated Quadrivalent Influenza
Vaccine Administered Concomitantly with a 23-Valent Pneumococcal Polysaccharide Vaccine in Adults Aged
60 Years and Older. Vaccines (Basel) 2024; 12(8).
157.
Nakafero G, Grainge MJ, Card T, Mallen CD, Nguyen Van-Tam JS, Abhishek A. Uptake and safety
of pneumococcal vaccination in adults with immune mediated inflammatory diseases: a UK wide observational
study. Rheumatology (Oxford) 2024.
158.
Wierzbowski A, Pless R, Hildebrand KJ. Summary of the NACI Statement on Public Health Level
Recommendations on the Use of Pneumococcal Vaccines in Adults, Including the Use of 15-valent and 20valent Conjugate Vaccines. Can Commun Dis Rep 2023; 49(23): 81-6.
159.
Harboe ZB, Larsen MV, Ladelund S, et al. Incidence and risk factors for invasive pneumococcal
disease in HIV-infected and non-HIV-infected individuals before and after the introduction of combination
antiretroviral therapy: persistent high risk among HIV-infected injecting drug users. Clin Infect Dis 2014;
59(8): 1168-76.
160.
Hechter RC, Qian L, Tartof SY, et al. Vaccine safety in HIV-infected adults within the Vaccine Safety
Datalink Project. Vaccine 2019; 37(25): 3296-302.
161.
Gianella S, Anderson C, Chaillon A, et al. Impact of influenza and pneumococcal vaccines on HIV
persistence and immune dynamics during suppressive antiretroviral therapy. Aids 2024; 38(8): 1131-40.
162.
Altawalbeh SM, Wateska AR, Nowalk MP, et al. Pneumococcal Vaccination Strategies in 50-Year-
Olds to Decrease Racial Disparities: A US Societal Perspective Cost-Effectiveness Analysis. Value Health 2024;
27(6): 721-9.
163.
Cantarero D, Ocaña D, Onieva-García MÁ, et al. Cost-utility analysis of the use of the 20-valent anti-
pneumococcal vaccine (PCV20) in adults older than 60 years in Spain. Vaccine 2023; 41(36): 5342-9.
164.
Danelian G, Burton L, Bayley T, et al. The impact and cost-effectiveness of pneumococcal
immunisation strategies for the elderly in England. Vaccine 2024; 42(18): 3838-50.
165.
de Boer PT, van Werkhoven CH, van Hoek AJ, et al. Higher-valency pneumococcal conjugate
vaccines in older adults, taking into account indirect effects from childhood vaccination: a cost-effectiveness
study for the Netherlands. BMC Med 2024; 22(1): 69.
166.
Gourzoulidis G, Barmpouni M, Kossyvaki V, Vietri J, Tzanetakos C. Health and economic outcomes
of 20-valent pneumococcal conjugate vaccine compared to 15-valent pneumococcal conjugate vaccine
strategies for adults in Greece. Front Public Health 2023; 11: 1229524.
44
Immunol 2024; 15: 1437267.
154.
Severance R, Schwartz H, Dagan R, et al. Safety, tolerability, and immunogenicity of V114, a 15-
valent pneumococcal conjugate vaccine, administered concomitantly with influenza vaccine in healthy adults
aged ≥50 years: a randomized phase 3 trial (PNEU-FLU). Hum Vaccin Immunother 2022; 18(1): 1-14.
155.
Cannon K, Cardona JF, Yacisin K, et al. Safety and immunogenicity of a 20-valent pneumococcal
conjugate vaccine coadministered with quadrivalent influenza vaccine: A phase 3 randomized trial. Vaccine
2023; 41(13): 2137-46.
156.
Zhu Z, Sun J, Xie Y, et al. Immunogenicity and Safety of an Inactivated Quadrivalent Influenza
Vaccine Administered Concomitantly with a 23-Valent Pneumococcal Polysaccharide Vaccine in Adults Aged
60 Years and Older. Vaccines (Basel) 2024; 12(8).
157.
Nakafero G, Grainge MJ, Card T, Mallen CD, Nguyen Van-Tam JS, Abhishek A. Uptake and safety
of pneumococcal vaccination in adults with immune mediated inflammatory diseases: a UK wide observational
study. Rheumatology (Oxford) 2024.
158.
Wierzbowski A, Pless R, Hildebrand KJ. Summary of the NACI Statement on Public Health Level
Recommendations on the Use of Pneumococcal Vaccines in Adults, Including the Use of 15-valent and 20valent Conjugate Vaccines. Can Commun Dis Rep 2023; 49(23): 81-6.
159.
Harboe ZB, Larsen MV, Ladelund S, et al. Incidence and risk factors for invasive pneumococcal
disease in HIV-infected and non-HIV-infected individuals before and after the introduction of combination
antiretroviral therapy: persistent high risk among HIV-infected injecting drug users. Clin Infect Dis 2014;
59(8): 1168-76.
160.
Hechter RC, Qian L, Tartof SY, et al. Vaccine safety in HIV-infected adults within the Vaccine Safety
Datalink Project. Vaccine 2019; 37(25): 3296-302.
161.
Gianella S, Anderson C, Chaillon A, et al. Impact of influenza and pneumococcal vaccines on HIV
persistence and immune dynamics during suppressive antiretroviral therapy. Aids 2024; 38(8): 1131-40.
162.
Altawalbeh SM, Wateska AR, Nowalk MP, et al. Pneumococcal Vaccination Strategies in 50-Year-
Olds to Decrease Racial Disparities: A US Societal Perspective Cost-Effectiveness Analysis. Value Health 2024;
27(6): 721-9.
163.
Cantarero D, Ocaña D, Onieva-García MÁ, et al. Cost-utility analysis of the use of the 20-valent anti-
pneumococcal vaccine (PCV20) in adults older than 60 years in Spain. Vaccine 2023; 41(36): 5342-9.
164.
Danelian G, Burton L, Bayley T, et al. The impact and cost-effectiveness of pneumococcal
immunisation strategies for the elderly in England. Vaccine 2024; 42(18): 3838-50.
165.
de Boer PT, van Werkhoven CH, van Hoek AJ, et al. Higher-valency pneumococcal conjugate
vaccines in older adults, taking into account indirect effects from childhood vaccination: a cost-effectiveness
study for the Netherlands. BMC Med 2024; 22(1): 69.
166.
Gourzoulidis G, Barmpouni M, Kossyvaki V, Vietri J, Tzanetakos C. Health and economic outcomes
of 20-valent pneumococcal conjugate vaccine compared to 15-valent pneumococcal conjugate vaccine
strategies for adults in Greece. Front Public Health 2023; 11: 1229524.
44