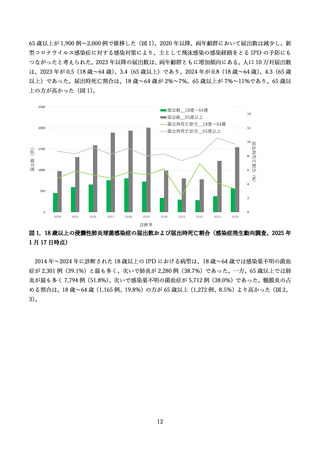
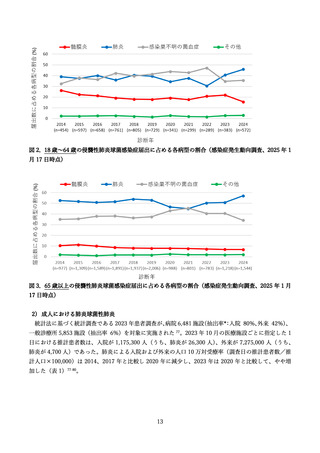
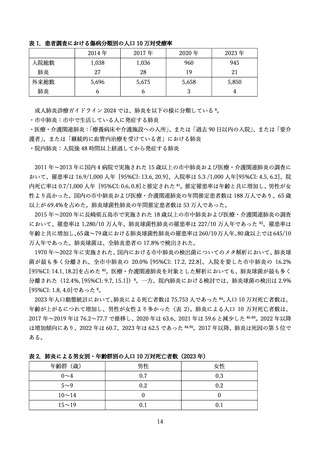
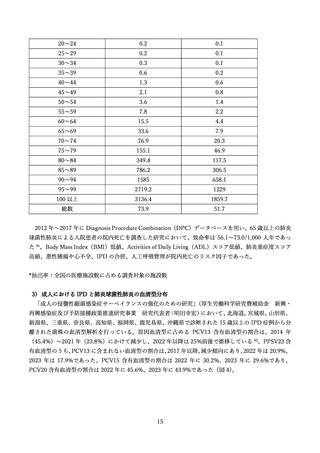
よむ、つかう、まなぶ。
10参考資料1-2 成人用肺炎球菌ワクチンファクトシート[4.9MB] (37 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_70339.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会(第64回 2/12)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
Assay for Detecting 11 Additional Streptococcus pneumoniae Serotype-specific Polysaccharides in Human
Urine. Clin Infect Dis 2020; 71(9): e430-e8.
52.
Pride MW, Huijts SM, Wu K, et al. Validation of an immunodiagnostic assay for detection of 13
Streptococcus pneumoniae serotype-specific polysaccharides in human urine. Clin Vaccine Immunol 2012;
19(8): 1131-41.
53.
Adegbola RA, Obaro SK, Biney E, Greenwood BM. Evaluation of Binax now Streptococcus
pneumoniae urinary antigen test in children in a community with a high carriage rate of pneumococcus.
Pediatr Infect Dis J 2001; 20(7): 718-9.
54.
Hamer DH, Egas J, Estrella B, MacLeod WB, Griffiths JK, Sempertegui F. Assessment of the Binax
NOW Streptococcus pneumoniae urinary antigen test in children with nasopharyngeal pneumococcal carriage.
Clin Infect Dis 2002; 34(7): 1025-8.
55.
佐藤長人, 高柳, 倉島一喜, et al. 肺炎球菌尿中抗原迅速検出キットの有用性と抗原反応強度・持続
期間の検討. 2004; (42): 247-52.
56.
綿貫祐司, 高橋宏, 小倉高志, 宮沢直幹, 冨岡敏昭, 小田切繁樹. 肺炎球菌性呼吸器感染症迅速診断
における尿中抗原検査と喀痰グラム染色検査の有用性の検討. 感染症誌 2005; 79: 13-9.
57.
吉田佳成子, 陽子 篠, 草野英美子, et al. 肺炎球菌尿中抗原検出キットを用いた尿中抗原陽性持続期
間についての検討. 日呼吸会誌 2003; 41: 521-5.
58.
栁原克紀. 「肺炎球菌細胞壁抗原検査」に関して. モダンメディア 2011; 57: 207-10.
59.
Lee J, Yoon Y, Kim EJ, et al. 23-valent polysaccharide vaccine (PPSV23)-targeted serotype-specific
identification of Streptococcus pneumoniae using the loop-mediated isothermal amplification (LAMP)
method. PLoS One 2021; 16(2): e0246699.
60.
Takano C, Kuramochi Y, Seki M, et al. Molecular serotype-specific identification of Streptococcus
pneumoniae using loop-mediated isothermal amplification. Sci Rep 2019; 9(1): 19823.
61.
Tomita Y, Okamoto A, Yamada K, Yagi T, Hasegawa Y, Ohta M. A new microarray system to detect
Streptococcus pneumoniae serotypes. J Biomed Biotechnol 2011; 2011: 352736.
62.
Oishi T, Ishiwada N, Matsubara K, et al. Low opsonic activity to the infecting serotype in pediatric
patients with invasive pneumococcal disease. Vaccine 2013; 31(5): 845-9.
63.
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility
Testing: Clinical and Laboratory Standards Institute; 2024.
64.
Yanagihara K, Watanabe A, Aoki N, et al. Nationwide surveillance of bacterial respiratory pathogens
conducted by the surveillance committee of Japanese Society of Chemotherapy, the Japanese Association for
Infectious Diseases, and the Japanese Society for Clinical Microbiology in 2012: General view of the pathogens'
antibacterial susceptibility. J Infect Chemother 2017; 23(9): 587-97.
65.
大竹正悟, 島田智恵, 砂川富正, et al. 基幹定点医療機関と JANIS におけるペニシリン耐性肺炎球菌
感染症報告の推移. IASR 2023; 44: 16-7.
66.
Suga S, Chang B, Asada K, et al. Nationwide population-based surveillance of invasive pneumococcal
disease in Japanese children: Effects of the seven-valent pneumococcal conjugate vaccine. Vaccine 2015;
33(45): 6054-60.
37
Urine. Clin Infect Dis 2020; 71(9): e430-e8.
52.
Pride MW, Huijts SM, Wu K, et al. Validation of an immunodiagnostic assay for detection of 13
Streptococcus pneumoniae serotype-specific polysaccharides in human urine. Clin Vaccine Immunol 2012;
19(8): 1131-41.
53.
Adegbola RA, Obaro SK, Biney E, Greenwood BM. Evaluation of Binax now Streptococcus
pneumoniae urinary antigen test in children in a community with a high carriage rate of pneumococcus.
Pediatr Infect Dis J 2001; 20(7): 718-9.
54.
Hamer DH, Egas J, Estrella B, MacLeod WB, Griffiths JK, Sempertegui F. Assessment of the Binax
NOW Streptococcus pneumoniae urinary antigen test in children with nasopharyngeal pneumococcal carriage.
Clin Infect Dis 2002; 34(7): 1025-8.
55.
佐藤長人, 高柳, 倉島一喜, et al. 肺炎球菌尿中抗原迅速検出キットの有用性と抗原反応強度・持続
期間の検討. 2004; (42): 247-52.
56.
綿貫祐司, 高橋宏, 小倉高志, 宮沢直幹, 冨岡敏昭, 小田切繁樹. 肺炎球菌性呼吸器感染症迅速診断
における尿中抗原検査と喀痰グラム染色検査の有用性の検討. 感染症誌 2005; 79: 13-9.
57.
吉田佳成子, 陽子 篠, 草野英美子, et al. 肺炎球菌尿中抗原検出キットを用いた尿中抗原陽性持続期
間についての検討. 日呼吸会誌 2003; 41: 521-5.
58.
栁原克紀. 「肺炎球菌細胞壁抗原検査」に関して. モダンメディア 2011; 57: 207-10.
59.
Lee J, Yoon Y, Kim EJ, et al. 23-valent polysaccharide vaccine (PPSV23)-targeted serotype-specific
identification of Streptococcus pneumoniae using the loop-mediated isothermal amplification (LAMP)
method. PLoS One 2021; 16(2): e0246699.
60.
Takano C, Kuramochi Y, Seki M, et al. Molecular serotype-specific identification of Streptococcus
pneumoniae using loop-mediated isothermal amplification. Sci Rep 2019; 9(1): 19823.
61.
Tomita Y, Okamoto A, Yamada K, Yagi T, Hasegawa Y, Ohta M. A new microarray system to detect
Streptococcus pneumoniae serotypes. J Biomed Biotechnol 2011; 2011: 352736.
62.
Oishi T, Ishiwada N, Matsubara K, et al. Low opsonic activity to the infecting serotype in pediatric
patients with invasive pneumococcal disease. Vaccine 2013; 31(5): 845-9.
63.
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility
Testing: Clinical and Laboratory Standards Institute; 2024.
64.
Yanagihara K, Watanabe A, Aoki N, et al. Nationwide surveillance of bacterial respiratory pathogens
conducted by the surveillance committee of Japanese Society of Chemotherapy, the Japanese Association for
Infectious Diseases, and the Japanese Society for Clinical Microbiology in 2012: General view of the pathogens'
antibacterial susceptibility. J Infect Chemother 2017; 23(9): 587-97.
65.
大竹正悟, 島田智恵, 砂川富正, et al. 基幹定点医療機関と JANIS におけるペニシリン耐性肺炎球菌
感染症報告の推移. IASR 2023; 44: 16-7.
66.
Suga S, Chang B, Asada K, et al. Nationwide population-based surveillance of invasive pneumococcal
disease in Japanese children: Effects of the seven-valent pneumococcal conjugate vaccine. Vaccine 2015;
33(45): 6054-60.
37