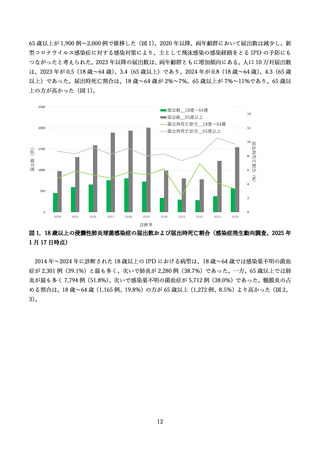
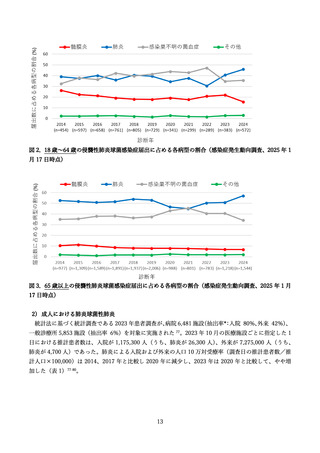
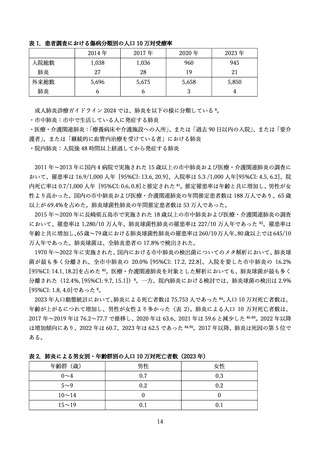
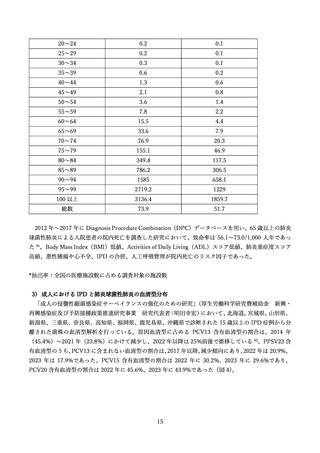
よむ、つかう、まなぶ。
10参考資料1-2 成人用肺炎球菌ワクチンファクトシート[4.9MB] (36 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_70339.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会(第64回 2/12)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
35.
Amin-Chowdhury Z, Aiano F, Mensah A, et al. Impact of the Coronavirus Disease 2019 (COVID-19)
Pandemic on Invasive Pneumococcal Disease and Risk of Pneumococcal Coinfection With Severe Acute
Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Prospective National Cohort Study, England. Clin Infect
Dis 2021; 72(5): e65-e75.
36.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a
systematic analysis. Lancet 2022; 399(10325): 629-55.
37.
World Health Organization. Global antimicrobial resistance and use surveillance system (GLASS)
report. 2022. https://www.who.int/publications/i/item/9789240062702 (accessed 2025/3/3).
38.
World Health Organization. Bacterial Priority Pathogens List. 2024.
https://www.who.int/publications/i/item/9789240093461 (accessed 2025/3/3).
39.
Li L, Ma J, Yu Z, Li M, Zhang W, Sun H. Epidemiological characteristics and antibiotic resistance
mechanisms of Streptococcus pneumoniae: An updated review. Microbiol Res 2023; 266: 127221.
40.
大竹正悟, 島田智恵, 砂川富正, et al. 基幹定点医療機関と JANIS におけるペニシリン耐性肺炎球菌
感染症報告の推移. 病原微生物検出情報 (IASR) 2023.
41.
国際的に脅威となる感染症対策の強化のための国際連携等関係閣僚会議. 薬剤耐性(AMR)対策ア
クションプラン
2023-2027. 2023. https://www.mhlw.go.jp/content/10900000/ap_honbun.pdf (accessed
2025/3/3).
42.
Kawaguchiya M, Urushibara N, Aung MS, et al. Clonal lineages and antimicrobial resistance of
nonencapsulated Streptococcus pneumoniae in the post-pneumococcal conjugate vaccine era in Japan. Int J
Infect Dis 2021; 105: 695-701.
43.
国立感染症研究所. 侵襲性肺炎球菌感染症 病原体検出マニュアル. 2021.
44.
Fukuyama H, Yamashiro S, Kinjo K, Tamaki H, Kishaba T. Validation of sputum Gram stain for
treatment of community-acquired pneumonia and healthcare-associated pneumonia: a prospective
observational study. BMC Infect Dis 2014; 14: 534.
45.
Centers for Disease Control and Prevention. Streptococcus Laboratory. Streptococcus pneumoniae
Detection and Serotyping Using PCR.
46.
Llull D, Lopez R, Garcia E. Characteristic signatures of the lytA gene provide a basis for rapid and
reliable diagnosis of Streptococcus pneumoniae infections. J Clin Microbiol 2006; 44(4): 1250-6.
47.
Simoes AS, Tavares DA, Rolo D, et al. lytA-based identification methods can misidentify
Streptococcus pneumoniae. Diagn Microbiol Infect Dis 2016; 85(2): 141-8.
48.
Hanage WP, Kaijalainen T, Herva E, Saukkoriipi A, Syrjanen R, Spratt BG. Using multilocus
sequence data to define the pneumococcus. J Bacteriol 2005; 187(17): 6223-30.
49.
Sadowy E, Hryniewicz W. Identification of Streptococcus pneumoniae and other Mitis streptococci:
importance of molecular methods. Eur J Clin Microbiol Infect Dis 2020; 39(12): 2247-56.
50.
Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in
adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled
clinical evaluation. J Clin Microbiol 2003; 41(7): 2810-3.
51.
Kalina WV, Souza V, Wu K, et al. Qualification and Clinical Validation of an Immunodiagnostic
36
Amin-Chowdhury Z, Aiano F, Mensah A, et al. Impact of the Coronavirus Disease 2019 (COVID-19)
Pandemic on Invasive Pneumococcal Disease and Risk of Pneumococcal Coinfection With Severe Acute
Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Prospective National Cohort Study, England. Clin Infect
Dis 2021; 72(5): e65-e75.
36.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a
systematic analysis. Lancet 2022; 399(10325): 629-55.
37.
World Health Organization. Global antimicrobial resistance and use surveillance system (GLASS)
report. 2022. https://www.who.int/publications/i/item/9789240062702 (accessed 2025/3/3).
38.
World Health Organization. Bacterial Priority Pathogens List. 2024.
https://www.who.int/publications/i/item/9789240093461 (accessed 2025/3/3).
39.
Li L, Ma J, Yu Z, Li M, Zhang W, Sun H. Epidemiological characteristics and antibiotic resistance
mechanisms of Streptococcus pneumoniae: An updated review. Microbiol Res 2023; 266: 127221.
40.
大竹正悟, 島田智恵, 砂川富正, et al. 基幹定点医療機関と JANIS におけるペニシリン耐性肺炎球菌
感染症報告の推移. 病原微生物検出情報 (IASR) 2023.
41.
国際的に脅威となる感染症対策の強化のための国際連携等関係閣僚会議. 薬剤耐性(AMR)対策ア
クションプラン
2023-2027. 2023. https://www.mhlw.go.jp/content/10900000/ap_honbun.pdf (accessed
2025/3/3).
42.
Kawaguchiya M, Urushibara N, Aung MS, et al. Clonal lineages and antimicrobial resistance of
nonencapsulated Streptococcus pneumoniae in the post-pneumococcal conjugate vaccine era in Japan. Int J
Infect Dis 2021; 105: 695-701.
43.
国立感染症研究所. 侵襲性肺炎球菌感染症 病原体検出マニュアル. 2021.
44.
Fukuyama H, Yamashiro S, Kinjo K, Tamaki H, Kishaba T. Validation of sputum Gram stain for
treatment of community-acquired pneumonia and healthcare-associated pneumonia: a prospective
observational study. BMC Infect Dis 2014; 14: 534.
45.
Centers for Disease Control and Prevention. Streptococcus Laboratory. Streptococcus pneumoniae
Detection and Serotyping Using PCR.
46.
Llull D, Lopez R, Garcia E. Characteristic signatures of the lytA gene provide a basis for rapid and
reliable diagnosis of Streptococcus pneumoniae infections. J Clin Microbiol 2006; 44(4): 1250-6.
47.
Simoes AS, Tavares DA, Rolo D, et al. lytA-based identification methods can misidentify
Streptococcus pneumoniae. Diagn Microbiol Infect Dis 2016; 85(2): 141-8.
48.
Hanage WP, Kaijalainen T, Herva E, Saukkoriipi A, Syrjanen R, Spratt BG. Using multilocus
sequence data to define the pneumococcus. J Bacteriol 2005; 187(17): 6223-30.
49.
Sadowy E, Hryniewicz W. Identification of Streptococcus pneumoniae and other Mitis streptococci:
importance of molecular methods. Eur J Clin Microbiol Infect Dis 2020; 39(12): 2247-56.
50.
Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in
adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled
clinical evaluation. J Clin Microbiol 2003; 41(7): 2810-3.
51.
Kalina WV, Souza V, Wu K, et al. Qualification and Clinical Validation of an Immunodiagnostic
36