よむ、つかう、まなぶ。
資料4 Ⅳ-112,140_メトトレキサート[1.5MB] (65 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00045.html |
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第67回 2/6)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
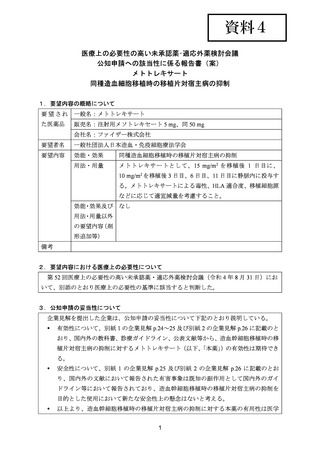
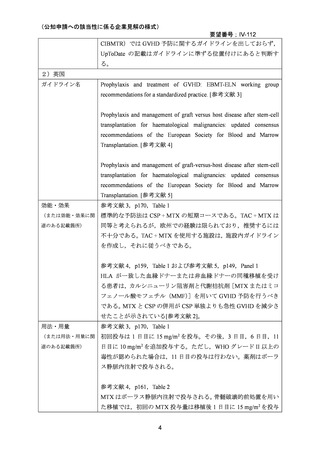
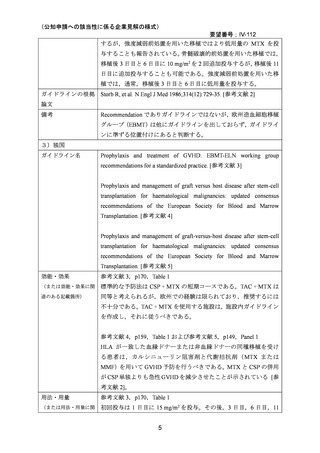
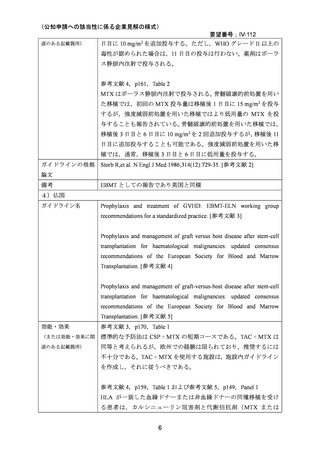
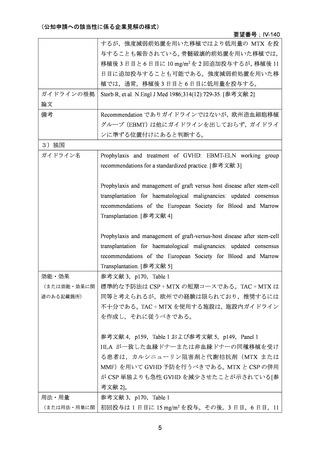
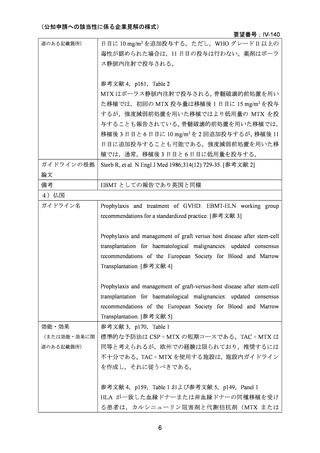
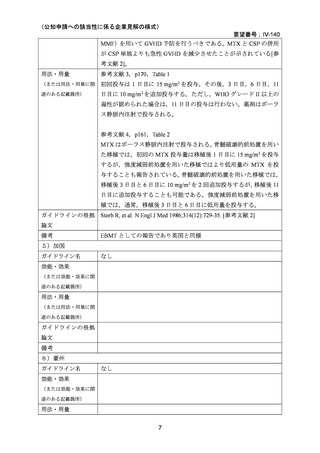
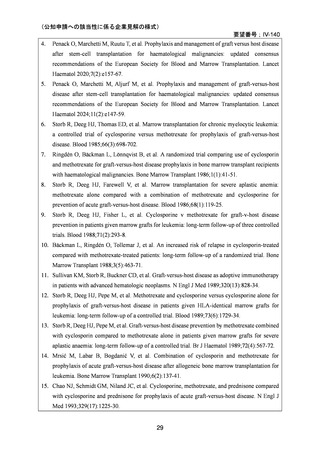
(公知申請への該当性に係る企業見解の様式)
要望番号;IV-140
16. Atkinson K, Biggs JC, Concannon A, et al. A prospective randomised trial of cyclosporin versus
methotrexate after HLA-identical sibling marrow transplantation for patients with acute
leukemia in first remission: analysis 2.5 years after last patient entry. Aust N Z J Med
1988;18(4):594-9.
17. Biggs JC, Atkinson K, Gillett E, et al. A randomized prospective trial comparing cyclosporine
and methotrexate given for prophylaxis of graft-versus-host disease after bone marrow
transplantation. Transplant Proc 1986;18(2):253-5.
18. Lee KH, Choi SJ, Lee JH, et al. Cyclosporine alone vs cyclosporine plus methotrexate for posttransplant immunosuppression after HLA-identical sibling bone marrow transplantation: a
randomized prospective study. Bone Marrow Transplant 2004;34(7):627-36.
19. Morishima Y, Morishita Y, Tanimoto M, et al. Low incidence of acute graft-versus-host disease
by the administration of methotrexate and cyclosporine in Japanese leukemia patients after bone
marrow transplantation from human leukocyte antigen compatible siblings; possible role of
genetic
homogeneity.
The
Nagoya
Bone
Marrow
Transplantation
Group.
Blood
1989;74(6):2252-6.
20. Holtan SG, Versluis J, Weisdorf DJ, et al. Optimizing Donor Choice and GVHD Prophylaxis in
Allogeneic Hematopoietic Cell Transplantation. J Clin Oncol 2021;39(5):373-85.
21. Galvin F, Freeman GJ, Razi-Wolf Z, et al. Effects of cyclosporin A, FK 506, and mycalamide A
on the activation of murine CD4+ T cells by the murine B7 antigen. Eur J Immunol
1993;23(1):283-6.
22. Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and
tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease
prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998;92(7):230314.
23. Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with
methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow
transplantation from unrelated donors. Blood 2000;96(6):2062-8.
24. Hamilton BK, Liu Y, Hemmer MT, et al. Inferior Outcomes with Cyclosporine and
Mycophenolate Mofetil after Myeloablative Allogeneic Hematopoietic Cell Transplantation.
Biol Blood Marrow Transplant 2019;25(9):1744-55.
25. Chhabra S, Liu Y, Hemmer MT, et al. Comparative Analysis of Calcineurin Inhibitor-Based
Methotrexate and Mycophenolate Mofetil-Containing Regimens for Prevention of Graft-versusHost Disease after Reduced-Intensity Conditioning Allogeneic Transplantation. Biol Blood
Marrow Transplant 2019;25(1):73-85.
26. Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as
GVHD prophylaxis after matched, related donor allogeneic HCT. Blood 2014;124(8):1372-7.
27. Bensinger W, Stem Cell Trialists’ Collaborative Group. Individual patient data meta-analysis of
30
要望番号;IV-140
16. Atkinson K, Biggs JC, Concannon A, et al. A prospective randomised trial of cyclosporin versus
methotrexate after HLA-identical sibling marrow transplantation for patients with acute
leukemia in first remission: analysis 2.5 years after last patient entry. Aust N Z J Med
1988;18(4):594-9.
17. Biggs JC, Atkinson K, Gillett E, et al. A randomized prospective trial comparing cyclosporine
and methotrexate given for prophylaxis of graft-versus-host disease after bone marrow
transplantation. Transplant Proc 1986;18(2):253-5.
18. Lee KH, Choi SJ, Lee JH, et al. Cyclosporine alone vs cyclosporine plus methotrexate for posttransplant immunosuppression after HLA-identical sibling bone marrow transplantation: a
randomized prospective study. Bone Marrow Transplant 2004;34(7):627-36.
19. Morishima Y, Morishita Y, Tanimoto M, et al. Low incidence of acute graft-versus-host disease
by the administration of methotrexate and cyclosporine in Japanese leukemia patients after bone
marrow transplantation from human leukocyte antigen compatible siblings; possible role of
genetic
homogeneity.
The
Nagoya
Bone
Marrow
Transplantation
Group.
Blood
1989;74(6):2252-6.
20. Holtan SG, Versluis J, Weisdorf DJ, et al. Optimizing Donor Choice and GVHD Prophylaxis in
Allogeneic Hematopoietic Cell Transplantation. J Clin Oncol 2021;39(5):373-85.
21. Galvin F, Freeman GJ, Razi-Wolf Z, et al. Effects of cyclosporin A, FK 506, and mycalamide A
on the activation of murine CD4+ T cells by the murine B7 antigen. Eur J Immunol
1993;23(1):283-6.
22. Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and
tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease
prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998;92(7):230314.
23. Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with
methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow
transplantation from unrelated donors. Blood 2000;96(6):2062-8.
24. Hamilton BK, Liu Y, Hemmer MT, et al. Inferior Outcomes with Cyclosporine and
Mycophenolate Mofetil after Myeloablative Allogeneic Hematopoietic Cell Transplantation.
Biol Blood Marrow Transplant 2019;25(9):1744-55.
25. Chhabra S, Liu Y, Hemmer MT, et al. Comparative Analysis of Calcineurin Inhibitor-Based
Methotrexate and Mycophenolate Mofetil-Containing Regimens for Prevention of Graft-versusHost Disease after Reduced-Intensity Conditioning Allogeneic Transplantation. Biol Blood
Marrow Transplant 2019;25(1):73-85.
26. Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as
GVHD prophylaxis after matched, related donor allogeneic HCT. Blood 2014;124(8):1372-7.
27. Bensinger W, Stem Cell Trialists’ Collaborative Group. Individual patient data meta-analysis of
30