よむ、つかう、まなぶ。
参考資料4 TERMS 第7版 (2 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_25755.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和4年度第4回 5/24)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
4
目次
5
1.背景 ··························································································
1
6
2.目的 ··························································································
1
7
3.用語の定義 ·················································································
2
8
4.関連組織 ····················································································
3
9
4.1.医療機関 ··············································································
3
10
4.2.特約店 ·················································································
3
11
4.3.TERMS 委員会 ·····································································
3
12
4.4.第三者評価機関 ·····································································
3
13
4.5.組織図 ·················································································
4
14
5.情報提供及び教育 ········································································
4
15
5.1.対象者 ·················································································
4
16
5.2.実施方法 ··············································································
4
17
6.登録 ··························································································
8
18
6.1.登録対象者 ···········································································
8
19
6.2.登録要件 ··············································································
8
20
6.3.登録手順 ·············································································· 10
21
6.3.1.登録申請 ········································································ 10
22
6.3.2.登録通知 ········································································ 10
23
6.4.登録情報 ·············································································· 11
24
6.4.1.藤本製薬株式会社登録情報 ················································ 11
25
6.4.2.医療機関登録情報 ···························································· 11
26
6.5.登録申請内容の確認 ······························································· 11
27
6.6.登録情報の変更 ····································································· 12
28
7.流通、処方及び調剤 ····································································· 13
29
7.1.流通 ···················································································· 13
30
7.2.処方 ···················································································· 13
31
7.3.調剤 ···················································································· 14
32
7.4.遵守状況の定期確認 ······························································· 14
33
7.5.処方及び調剤終了までの流れ ··················································· 15
34
7.6.本手順の運用状況の確認 ························································· 16
35
8.薬剤管理及び妊娠回避の徹底等 ······················································ 17
36
8.1.薬剤管理 ·············································································· 17
37
8.1.1.保管場所 ········································································ 17
38
8.1.2.数量管理 ········································································ 17
39
8.1.2.1.医療機関及び特約店の数量管理 ····································· 17
40
8.1.2.2.患者の数量管理 ·························································· 17
目次 1
- 2-
目次
5
1.背景 ··························································································
1
6
2.目的 ··························································································
1
7
3.用語の定義 ·················································································
2
8
4.関連組織 ····················································································
3
9
4.1.医療機関 ··············································································
3
10
4.2.特約店 ·················································································
3
11
4.3.TERMS 委員会 ·····································································
3
12
4.4.第三者評価機関 ·····································································
3
13
4.5.組織図 ·················································································
4
14
5.情報提供及び教育 ········································································
4
15
5.1.対象者 ·················································································
4
16
5.2.実施方法 ··············································································
4
17
6.登録 ··························································································
8
18
6.1.登録対象者 ···········································································
8
19
6.2.登録要件 ··············································································
8
20
6.3.登録手順 ·············································································· 10
21
6.3.1.登録申請 ········································································ 10
22
6.3.2.登録通知 ········································································ 10
23
6.4.登録情報 ·············································································· 11
24
6.4.1.藤本製薬株式会社登録情報 ················································ 11
25
6.4.2.医療機関登録情報 ···························································· 11
26
6.5.登録申請内容の確認 ······························································· 11
27
6.6.登録情報の変更 ····································································· 12
28
7.流通、処方及び調剤 ····································································· 13
29
7.1.流通 ···················································································· 13
30
7.2.処方 ···················································································· 13
31
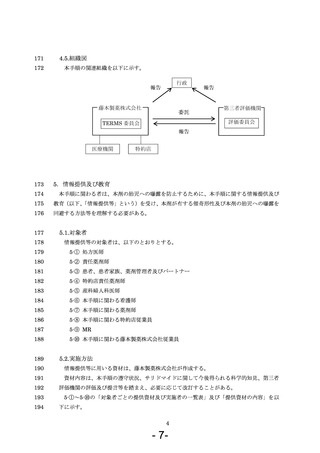
7.3.調剤 ···················································································· 14
32
7.4.遵守状況の定期確認 ······························································· 14
33
7.5.処方及び調剤終了までの流れ ··················································· 15
34
7.6.本手順の運用状況の確認 ························································· 16
35
8.薬剤管理及び妊娠回避の徹底等 ······················································ 17
36
8.1.薬剤管理 ·············································································· 17
37
8.1.1.保管場所 ········································································ 17
38
8.1.2.数量管理 ········································································ 17
39
8.1.2.1.医療機関及び特約店の数量管理 ····································· 17
40
8.1.2.2.患者の数量管理 ·························································· 17
目次 1
- 2-