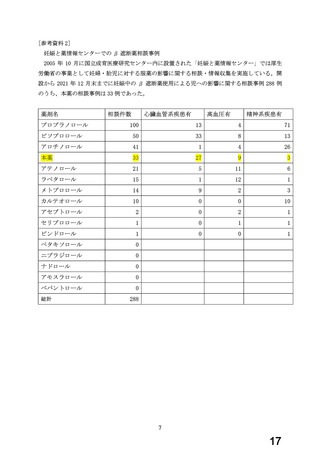
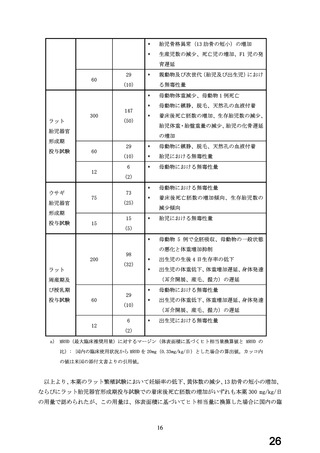
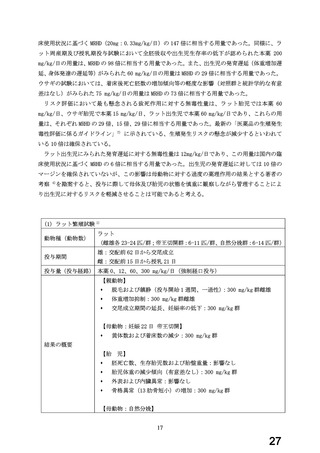
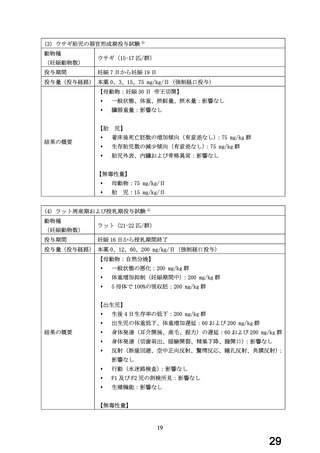
よむ、つかう、まなぶ。
資料1-2 カルベジロール 調査結果報告書及び添付文書[1.9MB] (23 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_38855.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和5年度第15回 3/26)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
postnatal period. Carvedilol should not be used during pregnancy unless
clearly necessary (that is if the potential benefit for the mother outweighs
the potential risk for the fetus/neonate). The treatment should be stopped 23 days before expected birth. If this is not possible the new-born has to be
monitored for the first 2-3 days of life.
5.3 Preclinical safety data
Carvedilol demonstrated no mutagenic or carcinogenic potential.
High doses of carvedilol impaired fertility and affected pregnancy in rats
(increased
resorptions).
development
were
also
Decreased
seen
in
fetal
rats.
weight
and
Embryotoxicity
delayed
skeletal
(increased
post-
implantation loss) occurred in rats and rabbits.
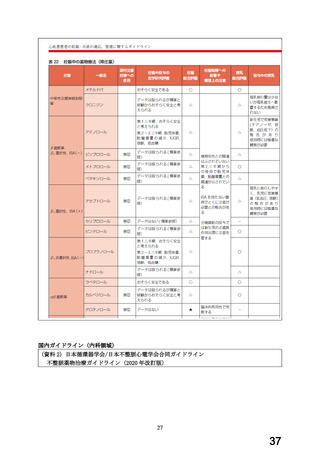
経口剤(加国)
(3) 製品名 APO-CARVEDILOL / APOTEX INC
効能・効果
INDICATIONS AND CLINICAL USE
APO-CARVEDILOL (carvedilol) is indicated for the treatment of mild, moderate
or severe heart failure of ischemic or non-ischemic origin to increase
survival and also, to reduce the combined risk of all-cause mortality and
cardiovascular or non-cardiovascular hospitalizations.
用法・用量
DOSAGE AND ADMINISTRATION
Recommended Dose and Dosage Adjustment
The recommended starting dose of APO-CARVEDILOL is 3.125 mg twice daily for
two weeks. If this dose is tolerated, it can then be increased to 6.25, 12.5
and 25 mg twice daily over successive intervals of at least 2 weeks. Patients
should be maintained on the highest tolerated dose. The maximum recommended
dose is 25 mg twice daily. The dose of APO-CARVEDILOL should not be increased
until symptoms of worsening heart failure or vasodilation have stabilized.
妊婦への
WARNINGS AND PRECAUTIONS
投与
Special Populations
Pregnant
Women:
There have been
no
clinical
studies carried out
to
specifically examine the use of carvedilol in pregnant women. Beta-blockers
reduce placental perfusion, which may result in intrauterine fetal death,
immature and premature deliveries. In addition, adverse effects (especially
hypoglycemia and bradycardia) may occur in the fetus and neonate. There is an
increased risk of cardiac and pulmonary complications in the neonate in the
postnatal period.
Animal reproduction studies have revealed no teratogenic potential for
carvedilol. Embryotoxicity was observed only after large doses in rabbits.
The relevance of these findings for humans is uncertain.
APO-CARVEDILOL should be used during pregnancy only if the potential benefit
justifies the potential risk to the fetus.
12
22
clearly necessary (that is if the potential benefit for the mother outweighs
the potential risk for the fetus/neonate). The treatment should be stopped 23 days before expected birth. If this is not possible the new-born has to be
monitored for the first 2-3 days of life.
5.3 Preclinical safety data
Carvedilol demonstrated no mutagenic or carcinogenic potential.
High doses of carvedilol impaired fertility and affected pregnancy in rats
(increased
resorptions).
development
were
also
Decreased
seen
in
fetal
rats.
weight
and
Embryotoxicity
delayed
skeletal
(increased
post-
implantation loss) occurred in rats and rabbits.
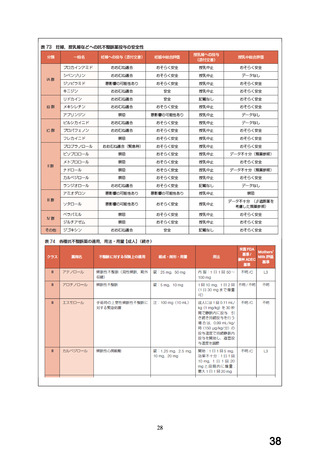
経口剤(加国)
(3) 製品名 APO-CARVEDILOL / APOTEX INC
効能・効果
INDICATIONS AND CLINICAL USE
APO-CARVEDILOL (carvedilol) is indicated for the treatment of mild, moderate
or severe heart failure of ischemic or non-ischemic origin to increase
survival and also, to reduce the combined risk of all-cause mortality and
cardiovascular or non-cardiovascular hospitalizations.
用法・用量
DOSAGE AND ADMINISTRATION
Recommended Dose and Dosage Adjustment
The recommended starting dose of APO-CARVEDILOL is 3.125 mg twice daily for
two weeks. If this dose is tolerated, it can then be increased to 6.25, 12.5
and 25 mg twice daily over successive intervals of at least 2 weeks. Patients
should be maintained on the highest tolerated dose. The maximum recommended
dose is 25 mg twice daily. The dose of APO-CARVEDILOL should not be increased
until symptoms of worsening heart failure or vasodilation have stabilized.
妊婦への
WARNINGS AND PRECAUTIONS
投与
Special Populations
Pregnant
Women:
There have been
no
clinical
studies carried out
to
specifically examine the use of carvedilol in pregnant women. Beta-blockers
reduce placental perfusion, which may result in intrauterine fetal death,
immature and premature deliveries. In addition, adverse effects (especially
hypoglycemia and bradycardia) may occur in the fetus and neonate. There is an
increased risk of cardiac and pulmonary complications in the neonate in the
postnatal period.
Animal reproduction studies have revealed no teratogenic potential for
carvedilol. Embryotoxicity was observed only after large doses in rabbits.
The relevance of these findings for humans is uncertain.
APO-CARVEDILOL should be used during pregnancy only if the potential benefit
justifies the potential risk to the fetus.
12
22