よむ、つかう、まなぶ。
資料1-2 カルベジロール 調査結果報告書及び添付文書[1.9MB] (22 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_38855.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和5年度第15回 3/26)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
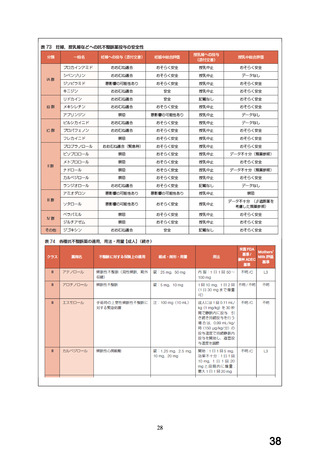
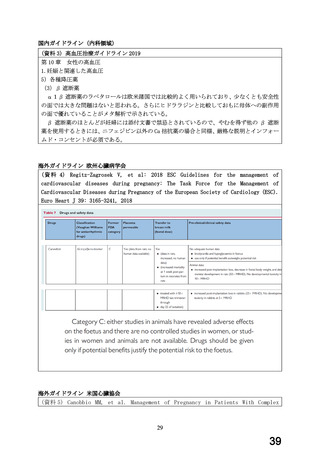
Thereafter, the treatment is continued at the dose 25 mg/day. If necessary,
the dose may be further increased gradually at intervals of two weeks or more
rarely.
Chronic stable angina pectoris:
A twice-daily regimen is recommended.
Adults:
The recommended initial dosage is 12.5 mg twice a day for the first two days.
Thereafter, the treatment is continued at the dose 25 mg twice a day. If
necessary, the dose may be further increased gradually at intervals of two
weeks or more rarely to the recommended maximum dose of 100 mg a day divided
into two doses (twice daily).
Heart Failure:
Carvedilol is given in moderate to severe heart failure in addition to
conventional basic therapy with diuretics, ACE inhibitors, digitalis, and/or
vasodilators. The patient should be clinically stable (no change in NYHAclass, no hospitalisation due to heart failure) and the basic therapy must be
stabilized for at least 4 weeks prior to treatment.
Additionally the patient should have a reduced left ventricular ejection
fraction and heart rate should be > 50 bpm and systolic blood pressure > 85
mm Hg (see section 4.3).
The initial dose is 3.125 mg twice a day for two weeks. If this dose is
tolerated, the dose may be increased slowly with intervals of not less than
two weeks up to 6.25 mg twice a day, then up to 12.5 mg twice a day and finally
up to 25 mg twice a day. The dosage should be increased to the highest
tolerable level.
The recommended maximum dosage is 25 mg twice a day for patients with a body
weight of less than 85 kg, and 50 mg twice a day for patients with a body
weight above 85 kg, provided that the heart failure is not severe. A dose
increase to 50 mg twice daily should be performed carefully under close medical
supervision of the patient.
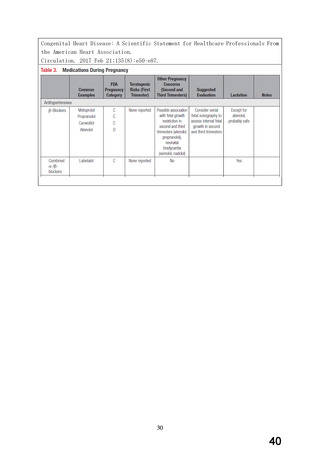
妊婦への
4. Clinical particulars
投与
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of carvedilol in pregnant women.
Studies in animals have shown reproductive toxicity (see section 5.3). The
potential risk for humans is unknown.
Beta-blockers reduce placental perfusion which may result in intrauterine
fetal death and immature and premature deliveries. In addition, adverse
reactions (especially hypoglycaemia, hypotension, bradycardia, respiratory
depression and hypothermia) may occur in the fetus and neonate. There is an
increased risk of cardiac and pulmonary complications in the neonate in the
11
21
the dose may be further increased gradually at intervals of two weeks or more
rarely.
Chronic stable angina pectoris:
A twice-daily regimen is recommended.
Adults:
The recommended initial dosage is 12.5 mg twice a day for the first two days.
Thereafter, the treatment is continued at the dose 25 mg twice a day. If
necessary, the dose may be further increased gradually at intervals of two
weeks or more rarely to the recommended maximum dose of 100 mg a day divided
into two doses (twice daily).
Heart Failure:
Carvedilol is given in moderate to severe heart failure in addition to
conventional basic therapy with diuretics, ACE inhibitors, digitalis, and/or
vasodilators. The patient should be clinically stable (no change in NYHAclass, no hospitalisation due to heart failure) and the basic therapy must be
stabilized for at least 4 weeks prior to treatment.
Additionally the patient should have a reduced left ventricular ejection
fraction and heart rate should be > 50 bpm and systolic blood pressure > 85
mm Hg (see section 4.3).
The initial dose is 3.125 mg twice a day for two weeks. If this dose is
tolerated, the dose may be increased slowly with intervals of not less than
two weeks up to 6.25 mg twice a day, then up to 12.5 mg twice a day and finally
up to 25 mg twice a day. The dosage should be increased to the highest
tolerable level.
The recommended maximum dosage is 25 mg twice a day for patients with a body
weight of less than 85 kg, and 50 mg twice a day for patients with a body
weight above 85 kg, provided that the heart failure is not severe. A dose
increase to 50 mg twice daily should be performed carefully under close medical
supervision of the patient.
妊婦への
4. Clinical particulars
投与
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of carvedilol in pregnant women.
Studies in animals have shown reproductive toxicity (see section 5.3). The
potential risk for humans is unknown.
Beta-blockers reduce placental perfusion which may result in intrauterine
fetal death and immature and premature deliveries. In addition, adverse
reactions (especially hypoglycaemia, hypotension, bradycardia, respiratory
depression and hypothermia) may occur in the fetus and neonate. There is an
increased risk of cardiac and pulmonary complications in the neonate in the
11
21