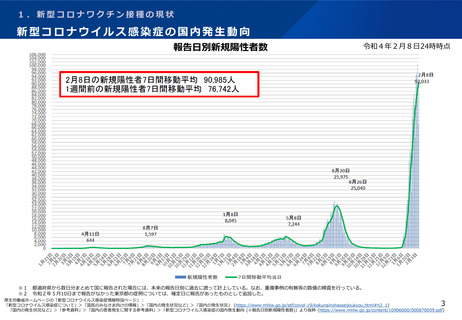
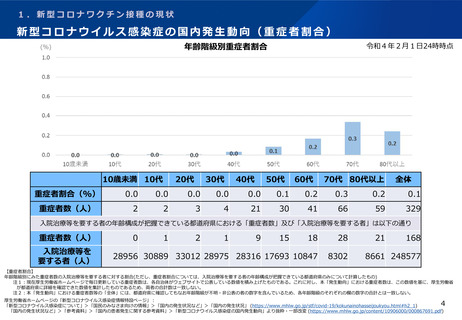
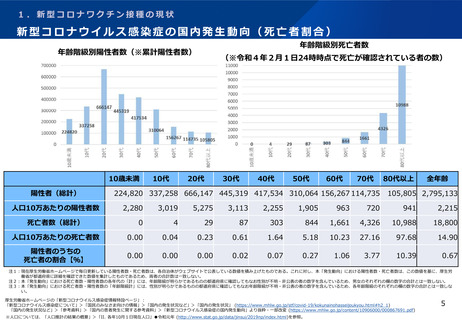
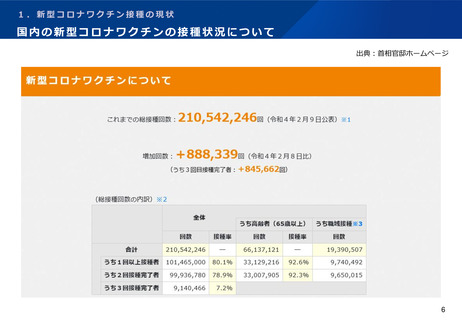
よむ、つかう、まなぶ。
03【資料1】新型コロナワクチンの接種について (87 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000192554_00019.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会(第30回 2/10)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
参考資料一覧(1/7)
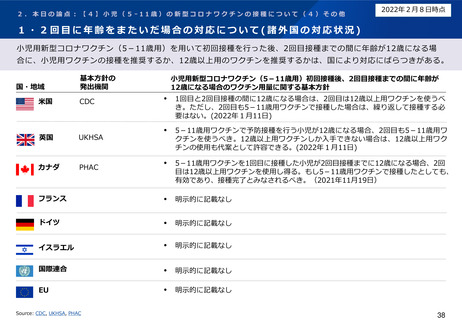
小児を対象とした新型コロナワクチンの諸外国の状況
米国
• Centers for Disease Control and Prevention.2021. COVID-19 Vaccination for Children 5-11 Years Old. [online] Available at: <https://www.cdc.gov/vaccines/covid19/planning/children.html> [Accessed 25 January 2021].
• Centers for Disease Control and Prevention. 2021. Coronavirus Disease 2019. [online] Available at: <https://www.cdc.gov/media/releases/2021/s1102-PediatricCOVID19Vaccine.html> [Accessed 25 January 2021].
英国
• GOV.UK. 2022. JCVI statement on COVID-19 vaccination of children and young people: 22 December 2021. [online] Available at:
<https://www.gov.uk/government/publications/jcvi-update-on-advice-for-covid-19-vaccination-of-children-and-young-people/jcvi-statement-on-covid-19-vaccination-ofchildren-and-young-people-22-december-2021> [Accessed 25 January 2022].
• NHS England. 2022. [online] Available at: <https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2021/12/C1524-updated-jvci-advice-for-the-vaccination-ofchildren-and-young-people.pdf> [Accessed 25 January 2022].
• GOV.UK. 2022. COVID-19: the green book, chapter 14a. [online] Available at: <https://www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a>
[Accessed 1 February 2022].
カナダ
• Health Canada. 2021. SUMMARY OF THE NATIONAL ADVISORY COMMITTEE ON IMMUNIZATION (NACI) STATEMENT OF NOVEMBER 19, 2021. [online] Available at:
<https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19vaccines/pfizer-biontech-10-mcg-children-5-11-years-age/summary/summary.pdf> [Accessed 25 January 2021].
フランス
• Ministère des Solidarités et de la Santé. 2021. La vaccination des enfants – Ministère des Solidarités et de la Santé. [online] Available at: <https://solidaritessante.gouv.fr/grands-dossiers/vaccin-covid-19/je-suis-un-particulier/article/la-vaccination-des-enfants> [Accessed 25 January 2021].
ドイツ
• Zusammengegencorona.de. 2021. [online] Available at: <https://www.zusammengegencorona.de/impfen/kinder/corona-schutzimpfung-ab-5-jahren/> [Accessed 25 January
2021].
イスラエル
• Ministry of Health. 2021. [online] Available at: <https://www.gov.il/en/departments/news/21112021-04> [Accessed 25 January 2021].
WHO
• WHO. 2022. Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing: Vaccines. [online] Available at:
<https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BNT162b2-2021.1> [Accessed 25 January 2021].
EMA
• European Medicines Agency. 2021. Comirnaty COVID-19 vaccine: EMA recommends approval for children aged 5 to 11 - European Medicines Agency. [online] Available at:
<https://www.ema.europa.eu/en/news/comirnaty-covid-19-vaccine-ema-recommends-approval-children-aged-5-11> [Accessed 25 January 2021].
87
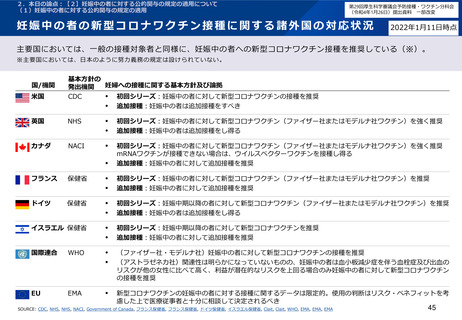
小児を対象とした新型コロナワクチンの諸外国の状況
米国
• Centers for Disease Control and Prevention.2021. COVID-19 Vaccination for Children 5-11 Years Old. [online] Available at: <https://www.cdc.gov/vaccines/covid19/planning/children.html> [Accessed 25 January 2021].
• Centers for Disease Control and Prevention. 2021. Coronavirus Disease 2019. [online] Available at: <https://www.cdc.gov/media/releases/2021/s1102-PediatricCOVID19Vaccine.html> [Accessed 25 January 2021].
英国
• GOV.UK. 2022. JCVI statement on COVID-19 vaccination of children and young people: 22 December 2021. [online] Available at:
<https://www.gov.uk/government/publications/jcvi-update-on-advice-for-covid-19-vaccination-of-children-and-young-people/jcvi-statement-on-covid-19-vaccination-ofchildren-and-young-people-22-december-2021> [Accessed 25 January 2022].
• NHS England. 2022. [online] Available at: <https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2021/12/C1524-updated-jvci-advice-for-the-vaccination-ofchildren-and-young-people.pdf> [Accessed 25 January 2022].
• GOV.UK. 2022. COVID-19: the green book, chapter 14a. [online] Available at: <https://www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a>
[Accessed 1 February 2022].
カナダ
• Health Canada. 2021. SUMMARY OF THE NATIONAL ADVISORY COMMITTEE ON IMMUNIZATION (NACI) STATEMENT OF NOVEMBER 19, 2021. [online] Available at:
<https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19vaccines/pfizer-biontech-10-mcg-children-5-11-years-age/summary/summary.pdf> [Accessed 25 January 2021].
フランス
• Ministère des Solidarités et de la Santé. 2021. La vaccination des enfants – Ministère des Solidarités et de la Santé. [online] Available at: <https://solidaritessante.gouv.fr/grands-dossiers/vaccin-covid-19/je-suis-un-particulier/article/la-vaccination-des-enfants> [Accessed 25 January 2021].
ドイツ
• Zusammengegencorona.de. 2021. [online] Available at: <https://www.zusammengegencorona.de/impfen/kinder/corona-schutzimpfung-ab-5-jahren/> [Accessed 25 January
2021].
イスラエル
• Ministry of Health. 2021. [online] Available at: <https://www.gov.il/en/departments/news/21112021-04> [Accessed 25 January 2021].
WHO
• WHO. 2022. Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing: Vaccines. [online] Available at:
<https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BNT162b2-2021.1> [Accessed 25 January 2021].
EMA
• European Medicines Agency. 2021. Comirnaty COVID-19 vaccine: EMA recommends approval for children aged 5 to 11 - European Medicines Agency. [online] Available at:
<https://www.ema.europa.eu/en/news/comirnaty-covid-19-vaccine-ema-recommends-approval-children-aged-5-11> [Accessed 25 January 2021].
87