よむ、つかう、まなぶ。
資料5-2 Ⅳ-122 ゲムシタビン[1.4MB] (31 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00044.html |
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第66回 12/12)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
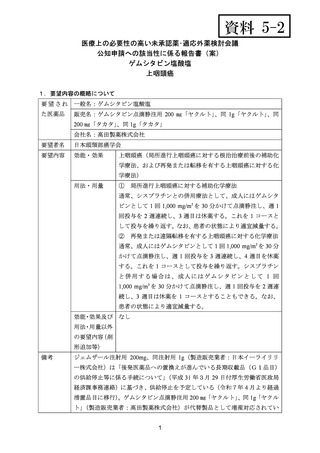
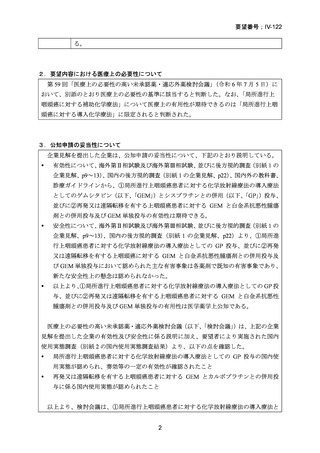
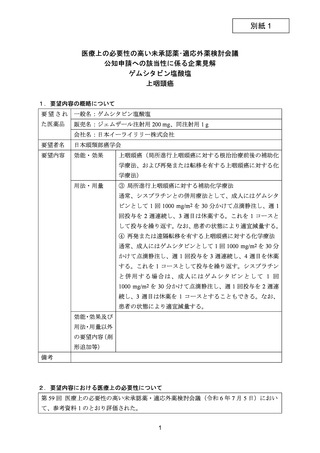
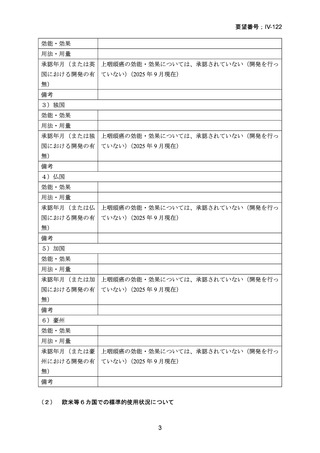
要望番号;IV-122
disease. Ann Oncol. 2023;34(3): 247-50.
5) 日本臨床腫瘍学会 編. 新臨床腫瘍学改訂第 7 版. 南江堂; 2024.
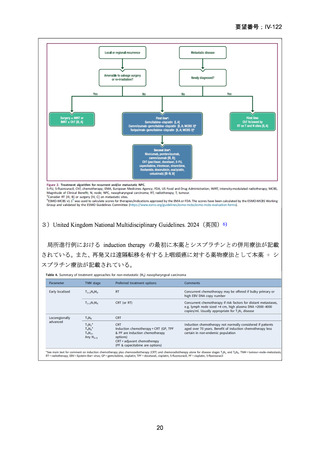
6) Homer JJ, Winter SC, Abbey EC, et al. Head and Neck Cancer: United Kingdom National
Multidisciplinary Guidelines, Sixth Edition. J Laryngol Otol. 2024;138(S1): S1-S224.
7) Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin induction chemotherapy in
nasopharyngeal carcinoma. N Eng J Med. 2019;381(12): 1124-35.
8) Zhang Y, Chen L, Hu GQ, et al. Final overall survival analysis of gemcitabine and cisplatin
induction chemotherapy in nasopharyngeal carcinoma: A multicenter, randomized phase III
trial. J Clin Oncol. 2022;40(22): 2420-5.
9) Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin
in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label,
phase 3 trial. Lancet. 2016;388(10054): 1883-92.
10) Zhang L, Zhang Y, Huang PY, et al. Phase II clinical study of gemcitabine in the treatment of
patients with advanced nasopharyngeal carcinoma after the failure of platinum-based
chemotherapy. Cancer Chemother Pharmacol. 2008;61(1): 33-8.
11) Jin Y, Cai XY, Shi YX, et al. Comparison of five cisplatin-based regimens frequently used as the
first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol.
2012;138(10): 1717-25.
12) Yang H, Lu Y, Xu Z, et al. Gemcitabine plus platinum versus docetaxel plus platinum as firstline therapy for metastatic nasopharyngeal carcinoma: A randomized clinical study. Saudi J Med
Med Sci. 2021;9(2): 125-34.
13) Hong S, Zhang Y, Yu G, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin as
first-line therapy for recurrent or metastatic nasopharyngeal carcinoma: Final overall survival
analysis of GEM20110714 phase III study. J Clin Oncol. 2021;39(29): 3273-82.
14) Jiromaru R, Nakagawa T, Yasumatsu R. Advanced nasopharyngeal carcinoma: Current and
emerging treatment options. Cancer Manag Res. 2022;14: 2681-9.
15) Ng WT, Corry J, Langendijk JA, et al. Current management of stage IV nasopharyngeal
carcinoma without distant metastasis. Cancer Treat Rev. 2020;85: 101995.
16) Liu T, Dai S, Zhang H, et al. The best choice of induction chemotherapy for patients with locally
advanced nasopharyngeal carcinoma: Bayesian network meta-analysis. Head Neck. 2022;44(2): 518-29.
17) Wang BC, Kuang BH, Liu XX, et al. Induction chemotherapy in locoregionally advanced
nasopharyngeal carcinoma: A systematic review and meta-analysis. Front Oncol. 2022;12:
927510.
18) Wu Q, Li S, Liu J, et al. Optimal induction chemotherapy regimen for locoregionally advanced
nasopharyngeal carcinoma: an update Bayesian network meta-analysis. Eur Arch
Otorhinolaryngol. 2022;279(11): 5057-69.
19) DeVita VT Jr., Lawrence TS, Rosenberg SA. Devita, Hellman, and Rosenberg's Cancer:
27
disease. Ann Oncol. 2023;34(3): 247-50.
5) 日本臨床腫瘍学会 編. 新臨床腫瘍学改訂第 7 版. 南江堂; 2024.
6) Homer JJ, Winter SC, Abbey EC, et al. Head and Neck Cancer: United Kingdom National
Multidisciplinary Guidelines, Sixth Edition. J Laryngol Otol. 2024;138(S1): S1-S224.
7) Zhang Y, Chen L, Hu GQ, et al. Gemcitabine and cisplatin induction chemotherapy in
nasopharyngeal carcinoma. N Eng J Med. 2019;381(12): 1124-35.
8) Zhang Y, Chen L, Hu GQ, et al. Final overall survival analysis of gemcitabine and cisplatin
induction chemotherapy in nasopharyngeal carcinoma: A multicenter, randomized phase III
trial. J Clin Oncol. 2022;40(22): 2420-5.
9) Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin
in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label,
phase 3 trial. Lancet. 2016;388(10054): 1883-92.
10) Zhang L, Zhang Y, Huang PY, et al. Phase II clinical study of gemcitabine in the treatment of
patients with advanced nasopharyngeal carcinoma after the failure of platinum-based
chemotherapy. Cancer Chemother Pharmacol. 2008;61(1): 33-8.
11) Jin Y, Cai XY, Shi YX, et al. Comparison of five cisplatin-based regimens frequently used as the
first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol.
2012;138(10): 1717-25.
12) Yang H, Lu Y, Xu Z, et al. Gemcitabine plus platinum versus docetaxel plus platinum as firstline therapy for metastatic nasopharyngeal carcinoma: A randomized clinical study. Saudi J Med
Med Sci. 2021;9(2): 125-34.
13) Hong S, Zhang Y, Yu G, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin as
first-line therapy for recurrent or metastatic nasopharyngeal carcinoma: Final overall survival
analysis of GEM20110714 phase III study. J Clin Oncol. 2021;39(29): 3273-82.
14) Jiromaru R, Nakagawa T, Yasumatsu R. Advanced nasopharyngeal carcinoma: Current and
emerging treatment options. Cancer Manag Res. 2022;14: 2681-9.
15) Ng WT, Corry J, Langendijk JA, et al. Current management of stage IV nasopharyngeal
carcinoma without distant metastasis. Cancer Treat Rev. 2020;85: 101995.
16) Liu T, Dai S, Zhang H, et al. The best choice of induction chemotherapy for patients with locally
advanced nasopharyngeal carcinoma: Bayesian network meta-analysis. Head Neck. 2022;44(2): 518-29.
17) Wang BC, Kuang BH, Liu XX, et al. Induction chemotherapy in locoregionally advanced
nasopharyngeal carcinoma: A systematic review and meta-analysis. Front Oncol. 2022;12:
927510.
18) Wu Q, Li S, Liu J, et al. Optimal induction chemotherapy regimen for locoregionally advanced
nasopharyngeal carcinoma: an update Bayesian network meta-analysis. Eur Arch
Otorhinolaryngol. 2022;279(11): 5057-69.
19) DeVita VT Jr., Lawrence TS, Rosenberg SA. Devita, Hellman, and Rosenberg's Cancer:
27